Observations Axel Nimmerjahn Widening perspectives through plane windows and microscope lenses
Professor Axel Nimmerjahn grew up in a still-divided Germany; American soldiers were a familiar sight in his hometown in the southeastern region. The American cultural presence and leniency toward travel outside of Germany broadened Nimmerjahn’s perspective on life, inspiring a curiosity and inventiveness that drew him to science and Salk.
Inside Salk sat down with Nimmerjahn to hear about his journey to world renowned researcher.
Nimmerjahn studies the central nervous system with a focus on astrocytes—one of the most abundant cell types in the nervous system, responsible for regulating and protecting neurons—and microglia, the nervous system’s resident immune cells. In addition to enriching our understanding of important nervous system cells, he also creates new tools to study them, including miniature microscopes and computational and genetic techniques.
His innovative and collaborative nature has landed him roles as the director of Salk’s Waitt Advanced Biophotonics Center and the leader of a multimillion-dollar National Institutes of Health BRAIN (Brain Research Through Advancing Innovative Neurotechnologies®) Initiative Program. He has also been recognized with numerous awards over the years: a Whitehall Foundation Award, recognition as a Rita Allen Scholar, and National Institutes of Health honors and grants, such as a Director’s New Innovator Award, Exceptional Unconventional Research Enabling Knowledge Acceleration (EUREKA) Award, and multiple BRAIN Initiative Awards.
Reflecting Nimmerjahn’s many honors, he was promoted to full professor at Salk in April 2024—an exciting landmark in his ongoing scientific story.
What was it like growing up in a divided country?
AN: I grew up seeing military personnel and hearing stories about people risking and losing their lives trying to cross the inner-German border, traveling from East Germany to West Germany. It felt normal. But now, in retrospect, I realize how strange these things were to witness growing up.
We were relatively lucky to live where we did, though. My parents grew up during World War II and had to flee from East Germany to West Germany, which landed us in the western part of Germany where there were no restrictions on travel to other countries. And my parents really wanted to travel with me and my older brother. Looking back, those trips were very important because they opened my mind to all different cultures and ways of life. Doing and seeing all these different things helps you understand that things can be done in a lot of different ways, and it made me really curious and explorative in all parts of my life. I realized there’s no one way to do anything.
And how did you get from studying physics to studying the central nervous system?
AN: Physics was great, but I always had this interest in medicine or neuroscience that stuck with me through university. At the end of my physics degree, I had the opportunity to do my master’s and PhD at the Max Planck Institute for Medical Research, where I could combine both physics and neuroscience.
I joined the lab of someone who, like me, had a background in physics and then decided to move into another science. When I started my master’s program, I worked on solving physics problems on light distortion in order to create miniaturized microscopes to study processes in the brain.
We approached this problem of measuring the brain and other tissues through the lens of physics.
My PhD advisor had invented the very first miniaturized two-photon microscope during his postdoctoral research, and then I was his first graduate student. We worked together on the next generation of microscopes, and that was my entry point into neuroscience.
When did you begin to focus on glial cells?
AN: There weren’t a lot of people studying glial cells where I was. If anything, they were interested in using them as something to compare neurons to. When you look at their electrical activity, they seem silent. When you measure their chemical activity, they show slow, varied changes. Glial cells were used as the “other” cell type to contrast neurons with. Discoveries were made about glial cells in this process, but it was in pursuit of new information about neurons.
While everyone was coming up with these new ways to measure and study neurons, we continued coming across glial cells. A lot of people I worked with were like, “Oh yeah, don’t worry about those,” to which I responded, “What?!” Thankfully my advisor was generous enough to let me explore glial cells—and the imaging tools we’d been creating to study neurons were already perfect for studying them. That’s been the focus of my whole career since.
What made you decide to leave Germany?
AN: I had been reading about some researchers’ work at Stanford University during my graduate studies, then was lucky enough to head to Stanford with those investigators as my new mentors. Being there allowed me to dig deeper into the biology of what I was studying while not losing touch with all the technical development—and what I did there became the basis for my lab at Salk. Earlier, I was looking mostly at the brain, but since coming to Salk, my lab and I expanded to the spinal cord, and we have continued to look at both of these nervous system regions since then.
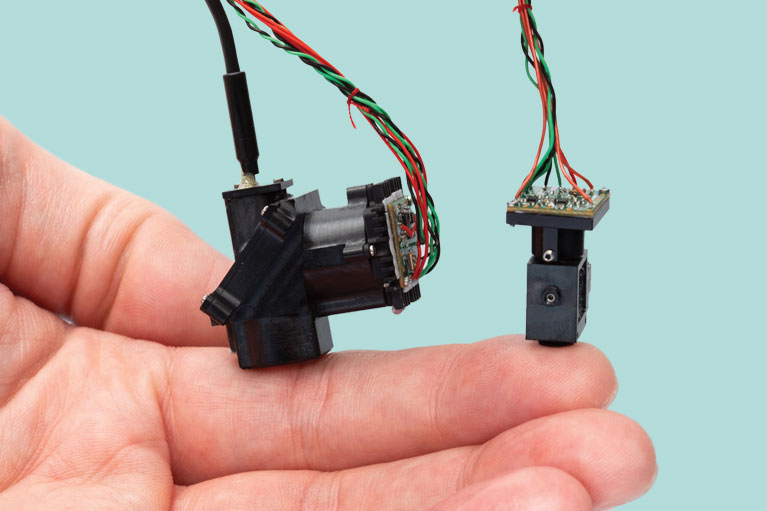
“We are continuing to develop wearable one-photon microscopes, which, like an iPhone, we can make more sophisticated with each generation.”
–Professor Axel Nimmerjahn
You’ve created a microscope small enough to fit on the tip of a human finger. Is the plan to keep making smaller and smaller microscopes?
AN: So, it’s a little more complicated—the microscopes I worked on as a graduate student were wearable two-photon microscopes (microscopes that capture images by exciting electrons using two massless particles of light called photons); the ones I worked on as a postdoc were wearable one-photon microscopes (microscopes that capture images by exciting electrons using one photon, which cannot image tissues as deeply as two-photon microscopes). We are continuing to develop wearable one-photon microscopes, which, like an iPhone, we can make more sophisticated with each generation. We can create additional capabilities, such as offering more color channels, and upgrade features like light sensitivity and image resolution.
We’re also hoping to establish a miniaturized three-photon microscope. One of the major challenges in the field is that light used for one- and two-photon imaging can only penetrate so far into a tissue. But there’s a lot of interesting stuff happening at tissue depths we cannot reach, so three-photon microscopes will be a huge help.
What sorts of questions can you ask and answer with your sophisticated microscopes?
AN: Prior to our microscopes, you could only measure small tissue regions at a time. Now, we can image multiple brain or spinal cord regions at once without sacrificing resolution. This is crucial because we know that whenever our bodies experience a stimulus—like touching a hot stove—there aren’t just a few neurons or a few cells stimulated by that. It’s actually distributed over a large area of many cells. And in order to understand how that information is encoded, and how that process transforms in diseases, we need imaging techniques that can capture larger areas and measure multiple cell types at once to see how everything is connected.
In addition to microscopes, you’ve also been helping to develop organoids. How is that going?
AN: Collaboration is one of my favorite things. We’ve been working with Salk Professor Rusty Gage, who pioneered these organoids (3D lab-grown models of human tissues), to take a step closer to human biology with our microscopes. We want to get closer to human biology so we can treat diseases, which is a very long process that starts with models like organoids and moves into clinical trials before finally ending with a usable drug.
Rusty’s lab has made a major step in creating human brain organoids that include both neurons and non-neuronal brain immune cells. Now we can see how neurons, immune cells, and pathogens or substances they produce interact. For example, if we catch a viral or bacterial infection, how do the immune cells in your brain react to that? Can we modulate that response? And what about genetic changes? We know that aging and Alzheimer’s disease are linked to genetic mutations that affect immune cell function in the brain. Could we potentially modulate their function to protect neurons from dysfunction?
Organoids are a way to study these questions, and while it’s not a perfect system, it’s a good step toward one.
What future research questions excite you right now?
AN: Our microscopes have really opened up so many possibilities and revealed so much new information. Recently, we discovered that astrocytes are involved in pain signaling in mice. I’m really excited to figure out how exactly they contribute to pain signaling, and eventually extend our findings into human cells. Hopefully, this can inspire new treatments for chronic pain. I am also really excited about the upcoming Neuroimmunology Initiative at Salk, which the NOMIS Foundation is funding to support our investigation into the interface between the nervous and immune systems across a number of disease contexts.
Some of the greatest challenges of our time—treating chronic pain, Alzheimer’s disease, and neuroimmune disorders like multiple sclerosis—require a team of investigators with expertise in all different areas. Going forward, when you look at papers in high-profile journals, you’re going to start gradually seeing more authors, institutions, and complex problems. We need to work as a team—and I’m happy to participate in a leading or supporting role just the same.
Featured Stories
 Getting to the root of Alzheimer’sSalk scientists are teaming up to understand brain aging. By collaborating across disciplines like genetics, neuroscience, and immunology, our researchers are uniquely positioned to lead us into a future of healthier aging and effective therapeutics for Alzheimer’s.
Getting to the root of Alzheimer’sSalk scientists are teaming up to understand brain aging. By collaborating across disciplines like genetics, neuroscience, and immunology, our researchers are uniquely positioned to lead us into a future of healthier aging and effective therapeutics for Alzheimer’s. Salk mourns the loss of Joan JacobsThe Salk Institute lost one of its greatest supporters and one of San Diego’s most generous philanthropists when Joan Jacobs died on May 6, 2024, in La Jolla, California. She was 91 years old.
Salk mourns the loss of Joan JacobsThe Salk Institute lost one of its greatest supporters and one of San Diego’s most generous philanthropists when Joan Jacobs died on May 6, 2024, in La Jolla, California. She was 91 years old. Axel Nimmerjahn— Widening perspectives through plane windows and microscope lensesInside Salk sat down with Nimmerjahn to hear how he went from curious child to world renowned researcher. Now a professor at Salk, he studies the central nervous system and designs new tools—like mini microscopes!—to study the system's various cell types.
Axel Nimmerjahn— Widening perspectives through plane windows and microscope lensesInside Salk sat down with Nimmerjahn to hear how he went from curious child to world renowned researcher. Now a professor at Salk, he studies the central nervous system and designs new tools—like mini microscopes!—to study the system's various cell types. Jálin Johnson— Enhancing equity and inclusion at Salk and beyondOver the course of nearly 30 years dedicated to advocacy work, Jálin B. Johnson has served those in need and has given a voice to the voiceless—a principle that has guided her throughout her career. This commitment continues to shape her work as she steps into the role of director of Salk’s Office of Equity & Inclusion.
Jálin Johnson— Enhancing equity and inclusion at Salk and beyondOver the course of nearly 30 years dedicated to advocacy work, Jálin B. Johnson has served those in need and has given a voice to the voiceless—a principle that has guided her throughout her career. This commitment continues to shape her work as she steps into the role of director of Salk’s Office of Equity & Inclusion. Lara Labarta-Bajo—An immunologist’s journey from Barcelona and ballet to the brainWhether dancing in Spain or surfing in San Diego, Lara Labarta-Bajo has always celebrated the power of the human body. Now a postdoctoral researcher in Associate Professor Nicola Allen’s lab at Salk, she studies how the immune system connects the body to the brain and how this relationship evolves as we age.
Lara Labarta-Bajo—An immunologist’s journey from Barcelona and ballet to the brainWhether dancing in Spain or surfing in San Diego, Lara Labarta-Bajo has always celebrated the power of the human body. Now a postdoctoral researcher in Associate Professor Nicola Allen’s lab at Salk, she studies how the immune system connects the body to the brain and how this relationship evolves as we age. Professor or partner? Luc Jansen’s switch from science to lawDashed dreams of medical school brought Jansen to Salk's campus back in 2003, where he simultaneously contemplated a career in scientific research and a career in big law. Though he decided on law in the end, Jansen's affection for Salk remains as he now hosts Salk gatherings in his New York City office and excitedly shares the enduring importance of basic science.
Professor or partner? Luc Jansen’s switch from science to lawDashed dreams of medical school brought Jansen to Salk's campus back in 2003, where he simultaneously contemplated a career in scientific research and a career in big law. Though he decided on law in the end, Jansen's affection for Salk remains as he now hosts Salk gatherings in his New York City office and excitedly shares the enduring importance of basic science. Rising Stars and DISCOVER programs provide new opportunities for trainees from underserved backgroundsThe Salk Institute recently hosted two inaugural events designed to enhance diversity within the scientific community: the Rising Stars Symposium and the Diverse Inclusive Scientific Community Offering a Vision for an Ecosystem Reimagined (DISCOVER) Symposium.
Rising Stars and DISCOVER programs provide new opportunities for trainees from underserved backgroundsThe Salk Institute recently hosted two inaugural events designed to enhance diversity within the scientific community: the Rising Stars Symposium and the Diverse Inclusive Scientific Community Offering a Vision for an Ecosystem Reimagined (DISCOVER) Symposium.






















































