Frontiers Getting to the root of Alzheimer’s
Salk Scientists are teaming up to understand brain aging
Annie Alessio was about to start her junior year at the University of San Diego when her mother, Carol, was diagnosed with early-onset Alzheimer’s disease.
“I remember it like it was yesterday—she walked through the door in her blue linen pantsuit, told me what happened, and said, ‘I’m going to beat this,’” says Annie. “If there was someone at the time who could have beat it, she would have been the one.”
Carol Alessio had always been an independent and strong-willed woman. In her youth, the spunky Midwestern girl often dreamed of a bigger and better life outside of her small town. At 18 years old, she bravely moved to Southern California, where she eventually met her husband, Mike, and immersed herself in San Diego’s business and political scenes.
It was on a trip back home in the summer of 2000 that Carol was suddenly injured in a fall. In the aftermath of the accident, family members began to recall a few other peculiar incidents from recent years and ultimately encouraged her to undergo some tests. The doctors soon discovered abnormalities in her brain scans, and the diagnosis came shortly thereafter.

“Alzheimer’s wasn’t on the forefront of everyone’s mind back then,” says Annie. “We knew it had to do with memory loss, but we didn’t know about the behavioral issues or the hallucinations. There weren’t websites or pamphlets explaining these things to us, so we didn’t know what to expect, and all of our lives just kind of stopped.”
Annie moved back home to help take care of her mother in the years before she was transferred to a nearby care facility. For the next 14 years, the family tried every medication and rehabilitation strategy her doctors suggested, but Carol’s mental and physical health continued to decline.
This perpetual struggle would be completely discouraging for most people, but Annie decided to channel her frustration into volunteer work. As a board member of the San Diego Alzheimer’s Association, she met with local doctors, led multiple fundraisers, and helped raise awareness of the disease.
August 2024 marked the 10th anniversary of Carol’s passing, and Annie hopes to honor her mother’s legacy with continued advocacy.
“I want to fight even harder on her behalf,” she says, “because I know that’s what she would do.”
Nearly all clinical research on Alzheimer’s has focused on amyloid plaques and tau tangles, but recent lab studies are pointing scientists in a new direction.

Barking up the wrong tree
Annie’s enthusiasm comes at a good time, as the landscape of Alzheimer’s research is currently experiencing a seismic shift. After decades of rather disappointing progress, scientists and clinicians are now reexamining the disease with new tools and a fresh perspective.
A main source of stagnation in Alzheimer’s research has been the overemphasis on amyloid plaques and tau tangles. These abnormal clumps of proteins in the brain were first observed by Alois Alzheimer himself in 1906, and thus became the defining biomarkers of the disease. For decades, these proteins were the focus of nearly all Alzheimer’s research, drug development, and clinical trials.
Many cases of the rare early-onset form of Alzheimer’s, like Carol’s, can be directly linked to gene mutations associated with amyloid and tau proteins. This was another early piece of evidence that got scientists and clinicians thinking these pathways must be the source of the pathology.
But over the years, it’s become clear that only a small subset of Alzheimer’s patients actually have these particular gene mutations and that not everyone who has these mutations goes on to develop the disease. So, there must be other factors at play.
“The field has really suffered from having this monolithic amyloid tau hypothesis,” says Salk Professor Rusty Gage, Vi and John Adler Chair for Research on Age-Related Neurodegenerative Disease. “It had such a hold on the field that researchers had little opportunity to study other ideas. That is now changing dramatically.”
The current sense is that these plaques and tangles represent specific genetic subtypes of Alzheimer’s or otherwise indicate a later stage of the disease that is likely much more difficult to treat. Scientists are now shifting their efforts to identify other sources of the disease—different genes, proteins, and pathways that, if treated early enough, could have much more success in improving patient outcomes.
“For many diseases, like cancer, the risk of developing the disease increases fairly steadily as we age, but there’s a different trajectory for the risk of Alzheimer’s, in that it’s nearly flat for most of our lives and then it takes off around age 70.”
–professor Gerald Shadel
Healthy Aging and Alzheimer’s
To get to the root of Alzheimer’s, Salk scientists are looking at the disease from all angles and incorporating the latest insights from healthy aging science. Through the Unlocking Healthy Aging Initiative, the Institute is expanding its efforts to understand aging on a more fundamental biological level. One major goal of these projects is to identify the cellular and molecular processes that contribute to aging in the brain.
Aging is the biggest risk factor for neurodegenerative disease, so much so that many assume one just inevitably comes with the other. And yet, everyone’s aging experience is different, and not everyone develops Alzheimer’s. So what makes the difference between mental fitness and frailty? What goes on in our brains as we age, and why does it sometimes lead to Alzheimer’s?
“For many diseases, like cancer, the risk of developing the disease increases fairly steadily as we age, but there’s a different trajectory for the risk of Alzheimer’s, in that it’s nearly flat for most of our lives and then it takes off around age 70,” says Gerald Shadel, professor and Audrey Geisel Chair in Biomedical Science at Salk. “In my mind, that means there’s a distinct set of aging-related phenomena that can happen around that point and trigger a quicker progression of Alzheimer’s.”
Shadel is the director of the San Diego Nathan Shock Center of Excellence in the Basic Biology of Aging, funded by the National Institute on Aging. The center was launched in 2020 to understand how intrinsic and environmental factors contribute to the heterogeneity of human aging. Its goal is to learn how these different factors affect each person’s aging trajectory so that personalized interventions can be developed to extend their “health span,” or the number of healthy years in their life.
“We all age, we all slow down and lose some abilities, and we all develop a higher risk of disease, but the issue is that for a long time, many people believed these things weren’t malleable,” says Shadel. “We’re now learning that if you understand the genetic and biochemical pathways of aging, you can target them therapeutically and actually slow the aging process—not because we want to live forever but because we want to be healthy for as much of our lives as possible.”
When Salk scientists discuss their aging research, they often refer to the idea of an “aging dashboard.” On each person’s dashboard, they imagine various gauges reporting the health status of different cells, tissues, organs, and even specific molecular pathways in their body. For some people, aging might look like a steady decline across all gauges, while others might have one or two areas in which aging is taking a bigger toll.
In a future in which these sorts of measurements could be consistently collected, artificial intelligence could analyze each person’s dashboard and predict their risk of developing an aging-related disease, such as Alzheimer’s. The key is being able to not just treat the symptoms of the disease as they emerge but proactively target the biggest sources of aging in that person to delay or even prevent the onset of disease altogether.
In the case of Alzheimer’s, scientists are finding that there are probably multiple potential starting points for the disease rather than one singular cause. This means the solution may come down to identifying which biological process (or processes) is being most affected by aging in each patient and targeting it as early as possible.
“If we could slow the major pathways contributing to someone’s neurological aging,” says Shadel, “even just delay them five or 10 years, that could make the difference between whether their final years are spent succumbing to Alzheimer’s, or if they can have that time with their loved ones and eventually pass in a less devastating manner.”

Aging damages mitochondria in the brain, causing energetic crises, inflammation, and neurodegeneration. Salk scientists are repurposing existing drugs to target these pathways and seeing promising results.
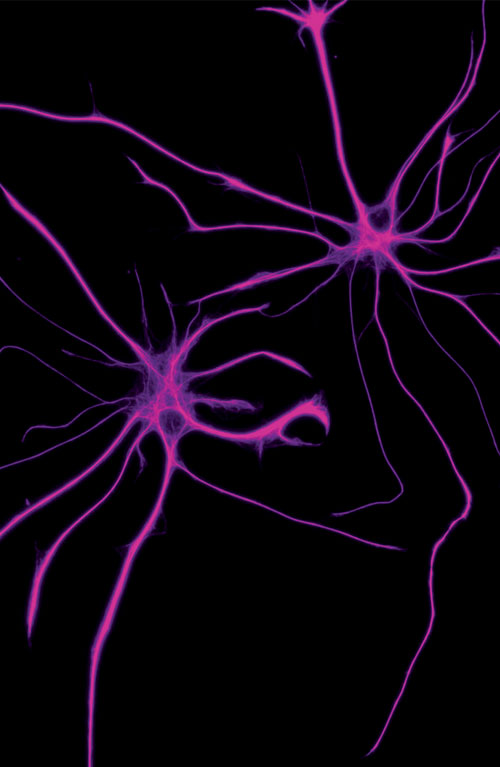
Salk scientists are finding that boosting specific proteins in astrocytes can restore synaptic function and memory. New ideas like these offer hope for better treatments.
Finding your roots
So what are these major brain aging pathways, and how can we slow them down to prevent disease? Gage, Shadel, and their Salk colleagues are hard at work figuring this out. In 2018, the team was awarded $19.2 million by the American Heart Association-Allen Initiative to launch a series of studies analyzing the interactions between proteins, genes, epigenetics, inflammation, and metabolism in Alzheimer’s and the aging brain.
“This team is purposely composed of faculty from all different disciplines because we knew that tackling Alzheimer’s and other forms of age-related cognitive decline would require us to think outside the box,” says Gage, who leads the initiative. “It’s an amazing group of people, and what’s fun is that we really all learn from each other and make each other better scientists.”
Gage and his team are pioneers of a modern research tool for modeling human brain aging in the lab. In this approach, the researchers collect skin samples from older adults, plate them on Petri dishes, and then use molecular tools to convert the skin cells directly into neurons. Recently, they advanced this technology to create 3D models called brain organoids, which include additional brain cell types like microglia and astrocytes to more accurately resemble human brain tissue. Their key breakthrough is finding a way to have these brain cells retain the molecular signatures of the patient’s age, making them invaluable for studying age-related diseases.
Using these models, Gage’s lab can compare the biology of brain cells from Alzheimer’s patients and age-matched healthy adults. In 2022, they published a study showing that many neurons derived from Alzheimer’s patients exhibit an age-related deterioration process called senescence. As these cells age, they become unable to produce enough energy to perform all their usual functions, eventually losing even the physical characteristics of neurons. In most cases, this much deterioration would cause cells to die, but senescent cells actually stay alive in this low-energy, zombie-like state and start to secrete inflammatory molecules. These leaky signals ultimately damage the surrounding tissue, exacerbating the problem and leading to cognitive decline.
To understand what triggers these cells to enter senescence, Gage has drawn on Shadel’s expertise in mitochondrial biology. Colloquially referred to as the “powerhouse of the cell,” mitochondria convert energy from the food we eat into chemical energy that our cells, tissues, and organs use to function. Human neurons require a lot of energy, so mitochondrial health is critical for normal brain function. However, aging can damage mitochondria in many ways, and, if left untreated, can lead to an energetic crisis, inflammation, and neurodegeneration.
Gage, Shadel, and other Salk scientists are now studying the various aging processes that weaken mitochondria in the brain. They are already seeing promising results from targeting these molecular pathways with existing drugs, offering hope for more effective treatments in the future.
In another line of research at Salk, scientists are looking at the ways that non-neuronal brain cells, called glia, can also contribute to Alzheimer’s. Interestingly, when scientists compare the gene expression profiles of brain cells from Alzheimer’s patients to those of healthy older adults, glial cells actually appear to be more affected by the disease than neurons.
Salk Associate Professor Nicola Allen is an expert on a subtype of glial cells called astrocytes, named for their star-like shape. Her lab is now uncovering the role that astrocytes play in Alzheimer’s disease.
Allen and her team have discovered that astrocytes are crucial for shaping communication across the brain. They mainly do this by guiding the formation and removal of synapses, where neurons meet and share electrochemical signals. Gene expression levels suggest that in Alzheimer’s patients, astrocytes are less able to create or strengthen synapses, while their propensity to remove synapses increases. This results in a destabilized and overly pruned synaptic network that ultimately disrupts brain communication.
Allen is now characterizing a class of proteins that astrocytes use to encourage the formation of new synaptic connections. Current experiments are testing whether re-expressing these proteins in the Alzheimer’s brain can restore synaptic function and delay disease progression.
Their initial results in mice are promising: Increasing the amount of these proteins in astrocytes restored the number of synapses in memory-related brain areas and improved animals’ performance in memory and spatial cognition tasks.
This study represents one of many cellular and molecular pathways that Allen’s team is exploring for the treatment of Alzheimer’s. Other lines of work in the lab are studying the relationship between astrocytes and neuroinflammation.
“Everyone’s taking different approaches, which is really exciting,” says Allen. “Some will work, and some won’t, but we’re going to learn a lot along the way.”
Another Salk scientist studying inflammation is Professor Susan Kaech, director of the NOMIS Center for Immunobiology and Microbial Pathogenesis and NOMIS Chair. Kaech’s research is centered on the immune system and cancer, but at Salk, she also lends her expertise to the study of aging and Alzheimer’s.
“Almost all of what we know about aging in humans is from sampling blood,” says Kaech, “and one of the biggest changes we see in our blood as we age is this large accumulation of memory T cells.”
When the body experiences an infection, the immune system produces an army of cells to find and kill the pathogen. It also produces memory T cells, whose job is to remember the pathogen so that the immune system can recognize and attack it even faster next time. But memory T cells are designed to respond not only to that one specific pathogen but also to others that are similar to it. This broad response is a smart strategy for fighting infection earlier in our lives, but as we accumulate more memory T cells with every infection, the net result is a large population of overresponsive immune cells across the body. Immunologists think this could be contributing to the chronically higher levels of inflammation seen in older adults.
“Long COVID showed people the lasting effects of that infection,” says Kaech, “but a lot of infections have long-lasting effects, and we still don’t know what they actually do to our tissues. There is probably a lot more immune involvement and possibly even autoimmunity in these neurodegenerative diseases than we’ve previously appreciated.”
Kaech first got involved in Alzheimer’s research at Salk to help study microglia, a type of immune cell found in the brain. But the more she spoke with her colleagues about their experiments, the more another question began to consume her.
“I was in these meetings, and I couldn’t help but think, ‘Man, all these people are studying the brain and behavior in animals that haven’t encountered any pathogens,’” she says, referring to the sterile environment that laboratory mice are traditionally housed in. “None of their findings take into account the lifetime of infections that we experience in the real world and how that may also contribute to inflammation and deterioration in the aging brain.”
Kaech is now pioneering a new experimental setup to introduce common infectious pathogens into the animals’ living environment in order to study the effects of infection on aging and brain health. Researchers across the Institute are eager to learn from her findings, but amidst all the excitement, Kaech notes one of the reasons these kinds of experiments aren’t done more often.
“Aging research is already expensive because the experiments take so long,” she says, “but it’s especially expensive and challenging to house mice in an environment that is considered biologically hazardous because of the presence of infectious pathogens.”
It’s obstacles like these that Salk’s Unlocking Healthy Aging Initiative is looking to overcome, by generating funding support to make this critical and cutting-edge research possible. The Institute also provides a unique environment that brings immunologists like Kaech into the same rooms as neuroscientists like Allen and Gage, which inspires these ideas in the first place.
Kaech’s work sets up a new paradigm for studying the biology of aging and Alzheimer’s against the natural backdrop of infection—and she’s not stopping there.
“Now that we’re establishing these controlled ways to introduce environmental factors into our experiments, we can systematically build on that to add things like exercise or Western diets, incrementally creating conditions that more accurately model our real world,” says Kaech. “Trying to study the effects of these environmental and lifestyle factors was previously considered too difficult or uncontrolled to be ‘hard science,’ but now we have the tools to do this in a systematic, scientific, and quantitative way.”
“Our studies are suggesting that there are different drivers of the disease in different people. This means there’s probably not going to be one drug that can treat everyone. We’re going to need different drugs for different people.”
–research professor Pamela Maher
A new Season of alzheimer’s science
With all this scientific innovation emerging across the Institute, Salk researchers are feeling hopeful about the future of Alzheimer’s treatments—but it may look a little different than we expected.
“Our studies are suggesting that there are different drivers of the disease in different people,” says Salk Research Professor Pamela Maher. “This means there’s probably not going to be one drug that can treat everyone. We’re going to need different drugs for different people.”
Maher was one of the first to take aging into account in Alzheimer’s drug discovery. She was able to identify a class of compounds known as geroneuroprotectors that slowed brain aging in mice and protected their cognitive function. Maher’s work has led to the development of several new drugs currently in clinical trials to treat Alzheimer’s. Her sense is that Alzheimer’s patients can likely be sorted into several groups based on biomarkers in their blood and that different classes of drugs could be prescribed based on which group one falls into.
“The good thing is, most of these aging pathways don’t have to be completely corrected or restored to make a difference,” says Gage.
When aging affects a specific molecular pathway in a person, he says, it’s not that this pathway has dropped to zero percent functionality and has to be recovered back to 100 percent. It may be that by a certain age, that process is already hovering around 50 percent in most people, but if things go lower than, say, 30 percent, that’s when Alzheimer’s may start to kick in. This means that if a medication or lifestyle change could target that specific pathway in a person and get it back up and running around even 40 percent, that might be enough to prevent the onset of the disease.
“It’s helpful that many of these fundamental aging pathways affect many aspects of our health at once,” says Shadel, “so just by delaying aging, you’ll likely delay many different things that could go wrong in the brain.”
While there is much work left to be done, this new perspective on Alzheimer’s research is finally paving the way to understanding its various root causes and how each can be best treated.
“I am confident that we will see disease-modifying medications for dementia in the near future,” says Gage. “And they won’t just decrease the rate of decline, which is what most current drugs aim to do—they will actually halt the disease progression, repair functional abilities, and improve patients’ quality of life.”
In this together
In reflecting on her family’s experience with Alzheimer’s, Annie Alessio feels a certain kinship with its researchers.
“I don’t know the scientists, and the scientists don’t know me, but we need to fight for each other,” she says.
“If things seem challenging, remember that what you’re doing is for the benefit of families like mine. We’re extremely grateful for what you do, and your efforts don’t go unnoticed. I’m hopeful that in my lifetime, there will be someone that gets this diagnosis and comes through it on the other side.”
Instead of treating the symptoms of Alzheimer’s as they emerge, Salk scientists are developing drugs to proactively target the aging processes that may trigger the disease in the first place.
Your support adds up—
Donate today to multiply innovations in Healthy Aging research.

Featured Stories
 Getting to the root of Alzheimer’sSalk scientists are teaming up to understand brain aging. By collaborating across disciplines like genetics, neuroscience, and immunology, our researchers are uniquely positioned to lead us into a future of healthier aging and effective therapeutics for Alzheimer’s.
Getting to the root of Alzheimer’sSalk scientists are teaming up to understand brain aging. By collaborating across disciplines like genetics, neuroscience, and immunology, our researchers are uniquely positioned to lead us into a future of healthier aging and effective therapeutics for Alzheimer’s. Salk mourns the loss of Joan JacobsThe Salk Institute lost one of its greatest supporters and one of San Diego’s most generous philanthropists when Joan Jacobs died on May 6, 2024, in La Jolla, California. She was 91 years old.
Salk mourns the loss of Joan JacobsThe Salk Institute lost one of its greatest supporters and one of San Diego’s most generous philanthropists when Joan Jacobs died on May 6, 2024, in La Jolla, California. She was 91 years old. Axel Nimmerjahn— Widening perspectives through plane windows and microscope lensesInside Salk sat down with Nimmerjahn to hear how he went from curious child to world renowned researcher. Now a professor at Salk, he studies the central nervous system and designs new tools—like mini microscopes!—to study the system's various cell types.
Axel Nimmerjahn— Widening perspectives through plane windows and microscope lensesInside Salk sat down with Nimmerjahn to hear how he went from curious child to world renowned researcher. Now a professor at Salk, he studies the central nervous system and designs new tools—like mini microscopes!—to study the system's various cell types. Jálin Johnson— Enhancing equity and inclusion at Salk and beyondOver the course of nearly 30 years dedicated to advocacy work, Jálin B. Johnson has served those in need and has given a voice to the voiceless—a principle that has guided her throughout her career. This commitment continues to shape her work as she steps into the role of director of Salk’s Office of Equity & Inclusion.
Jálin Johnson— Enhancing equity and inclusion at Salk and beyondOver the course of nearly 30 years dedicated to advocacy work, Jálin B. Johnson has served those in need and has given a voice to the voiceless—a principle that has guided her throughout her career. This commitment continues to shape her work as she steps into the role of director of Salk’s Office of Equity & Inclusion. Lara Labarta-Bajo—An immunologist’s journey from Barcelona and ballet to the brainWhether dancing in Spain or surfing in San Diego, Lara Labarta-Bajo has always celebrated the power of the human body. Now a postdoctoral researcher in Associate Professor Nicola Allen’s lab at Salk, she studies how the immune system connects the body to the brain and how this relationship evolves as we age.
Lara Labarta-Bajo—An immunologist’s journey from Barcelona and ballet to the brainWhether dancing in Spain or surfing in San Diego, Lara Labarta-Bajo has always celebrated the power of the human body. Now a postdoctoral researcher in Associate Professor Nicola Allen’s lab at Salk, she studies how the immune system connects the body to the brain and how this relationship evolves as we age. Professor or partner? Luc Jansen’s switch from science to lawDashed dreams of medical school brought Jansen to Salk's campus back in 2003, where he simultaneously contemplated a career in scientific research and a career in big law. Though he decided on law in the end, Jansen's affection for Salk remains as he now hosts Salk gatherings in his New York City office and excitedly shares the enduring importance of basic science.
Professor or partner? Luc Jansen’s switch from science to lawDashed dreams of medical school brought Jansen to Salk's campus back in 2003, where he simultaneously contemplated a career in scientific research and a career in big law. Though he decided on law in the end, Jansen's affection for Salk remains as he now hosts Salk gatherings in his New York City office and excitedly shares the enduring importance of basic science. Rising Stars and DISCOVER programs provide new opportunities for trainees from underserved backgroundsThe Salk Institute recently hosted two inaugural events designed to enhance diversity within the scientific community: the Rising Stars Symposium and the Diverse Inclusive Scientific Community Offering a Vision for an Ecosystem Reimagined (DISCOVER) Symposium.
Rising Stars and DISCOVER programs provide new opportunities for trainees from underserved backgroundsThe Salk Institute recently hosted two inaugural events designed to enhance diversity within the scientific community: the Rising Stars Symposium and the Diverse Inclusive Scientific Community Offering a Vision for an Ecosystem Reimagined (DISCOVER) Symposium.






















































