FRONTIERS The Science of Aging Salk scientists are solving the mysteries of optimal aging for the body and mind
Jeanne Calment, a Frenchwoman born in 1875, stayed physically active and mentally alert until passing away at almost 123 years of age. She outlived her daughter and her grandson and has been recognized as the longest-living human. She cycled regularly until she sustained a leg fracture at age 100; smoked a daily cigarette until 117; and enjoyed “considerable” quantities of chocolate until her death.
Aside from Calment, a handful of people worldwide lived well into their 110s. Researchers have scrutinized the habits of these supercentenarians, but such long-lived humans have surprisingly little in common, aside from the observation that women tend to live longer than men.
So what is their secret?
What we know about longevity so far: Minimizing smoking, obesity and overeating while maximizing exercise and social interactions seem to correspond to longer and healthier lives, but not always. While many factors have been touted as panaceas for extending life–everything from adhering to a Mediterranean diet to regularly imbibing red wine–a “fountain of youth” has remained stubbornly elusive.
“Aging is such a profound part of not only the human experience but all life on Earth,” says Salk Vice President/Chief Science Officer Martin Hetzer. “It’s one of the big, untapped opportunities in biomedical research, particularly around questions on what role exercise, nutrition and cognitive stimulation play in staying healthy throughout life. It is important not to forget that getting older also comes with benefits; we want to take a holistic view of human health at all ages and understand it from all angles.”
Scientists want to answer intriguing questions: Why are some people able to “age well,” trekking up mountain ranges or rafting through white water in their nineties, while others live just as long, disease-free, but grow inexplicably frail decades sooner? Worse yet, why does advanced age sometimes diminish cognitive ability or even lead to dementia?
In numerous diseases, age itself is the major risk factor. Cancer, Alzheimer’s, heart disease and many other afflictions become profoundly more likely the older we get. Aside from extending our life spans, scientists want to know how we can also extend our health during advanced age. What is emerging from research is that aging–loosely defined as a systems-wide deterioration of our cells, organs and genetic material that results in disease or damage–is a collective and complex process in the body.
“While we know that exercise and dietary restriction generally increase the lifespan of an organism, the molecular mechanisms are still unknown,” says Professor Jan Karlseder, director of the Paul F. Glenn Center for Biology of Aging Research at Salk. “It appears that many factors talk to each other and influence the whole system of the body in relation to aging. Here at Salk, we are well positioned to get to the bottom of this ‘crosstalk’ of aging because labs in different specialties are working closely together.”
Salk Institute researchers are forging new partnerships across fields–computer science, genetics, epigenetics, neuroscience, immunology and other areas of molecular biology–in order to spearhead ambitious studies to reveal how we can live longer and healthier. These scientists are diving into molecular, genetic, cellular and systems-wide processes in the body; peering into the fundamental workings of cells; and developing cutting-edge techniques to replace and repair failing organs and tissues. Together, Salk scientists are paving the way for a new view of age-related diseases and pointing to therapies to extend our healthy lifespan.
Telomeres–a ticking clock in cellular aging
Telomeres, the protective tips at the ends of chromosomes, are a focal point in the field of aging. The length of telomeres, which consist of repetitive pieces of DNA encased in proteins, is tied to a cell’s–and an organism’s–well-being.
When cells replicate their DNA and divide, a small portion of each telomere is whittled away. This shortening acts as a ticking clock for cells, prompting cells to stop dividing as a way to prevent duplicating damaged DNA. That is bad news when it comes to maintaining our lungs, skin, liver and several other organs, which need to be continually replenished by stem cells throughout our life. Fortunately, stem cells produce an enzyme called telomerase, which can partially counteract this telomere erosion. But even stem cells cannot completely escape this cell-division clock, so they eventually succumb to the consequences of telomere loss.
If we can stop telomere erosion, scientists reason, maybe we can add time to the ticking clock affecting the health of our cells.
“All of us have heard stories about a relative or neighbor who had a nightly shot of bourbon and a cigar into their nineties and were just fine,” says Salk Professor Vicki Lundblad, holder of the Becky and Ralph S. O’Connor Chair. “Those aren’t tall tales; some humans break all the so-called rules and live a healthy and long life. We want to know if telomeres shorten more slowly in these people, enabling them to live decades longer than someone else with the same lifestyle.”
Lundblad, who serves as associate director of Salk’s Glenn Center, has recently developed a new methodology to observe at a very high resolution how telomeres change in length. Using this technique, her group has uncovered a wide category of genes, not previously implicated in telomere biology, that alter the rate at which telomeres shorten. In addition, Lundblad has gained insights into mechanisms that contribute to the erosion
of telomeres.
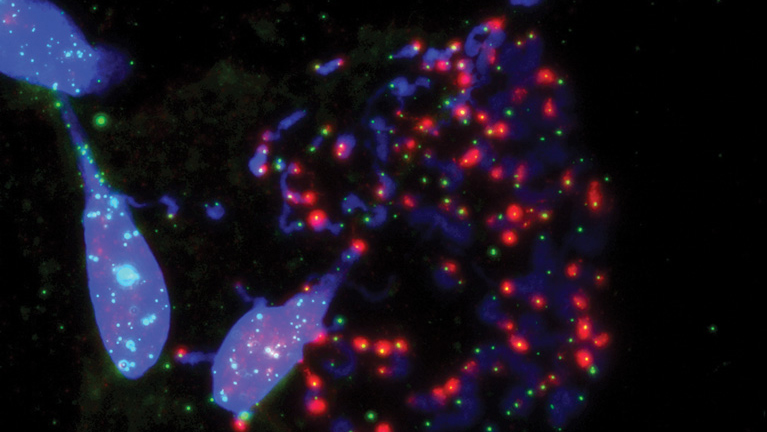
Salk researchers show how the disabled protection of ends of chromosomes (blue) called telomeres (green) during cell division prompts cell death. This happens when the telomere-protecting protein, TRF2, is inhibited.
“Our research suggests that a delicate balance between activities that elongate telomeres–such as telomerase–and the newly discovered erosion genes we have uncovered might dictate whether we can all live into our nineties, even with the nightly shot of bourbon,” Lundblad says.
Other research at Salk has shown that long telomeres aren’t necessarily always a good thing.
“In recent work we found that forcing cells to generate overly long telomeres can lead to stress and likely the initiation of cancer, indicating the need for a better understanding of the complexity of telomeres,” says Karlseder, who holds the Donald and Darlene Shiley Chair. If a telomere doesn’t have the right protein composition regardless of length, the cell suffers, indicating that lengthening telomeres to reverse signs of aging is not as simple as it might seem.
In addition to exploring how telomere function and other DNA repair activities change with age, Karlseder is collaborating with Salk Professor Gerald Shadel, an expert in mitochondria, the energy factories of the cell (See this issue’s “Observations” article to learn more about Shadel’s research). The duo wants to explore the potential crosstalk between cellular processes related to damage of a cell’s DNA and its mitochondria.
“Somehow signals from the nucleus, which recognizes that it’s aging through telomere shortening, get to the mitochondria to change the energy metabolism seen in aging cells,” says Karlseder. He is also exploring telomere activity in brain cells. “This is a completely unexplored area, a better understanding of which could inform treatments for a range of age-associated diseases, including dementia,” he says.
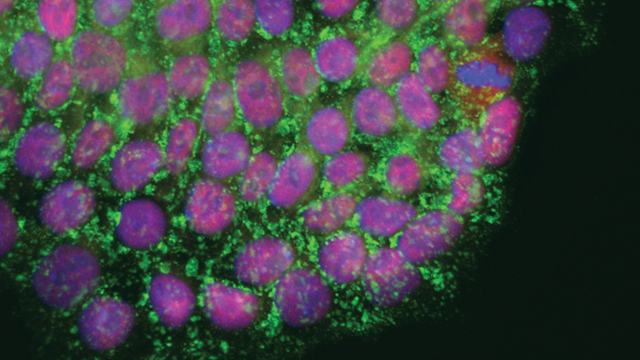
Salk scientists discover details into the optimal telomere length of human induced pluripotent stem cells (shown), a finding that could help advance stem cell-based therapies related to aging.
Deciphering–and reversing–signs of aging in organs
While one critical area of study in the field of aging involves studying telomeres in dividing cells, the body is full of cells that seldom replicate, such as those in the brain, heart, bones and pancreas. These long-lived cells have different pathways of aging, and must protect their DNA from damage and threats.
“It turns out that organs age at different rates, in part because they have unique strategies to maintain their integrity over time,” says Hetzer, who holds the Jesse and Caryl Philips Foundation Chair.
Hetzer studies changes in these cells–specifically their nuclei and mitochondria–as they age. He looks at the security membrane around the nucleus and why it malfunctions and mistakenly lets in dangerous molecules as the cell ages, as well as how components called long-lived proteins contribute to a cell’s health and degeneration over time.
Recently, Hetzer partnered with computer scientist and Salk Assistant Professor Saket Navlakha to determine people’s age based on biomarkers in their cells. Published in Genome Biology in December 2018, the study sampled a type of long-lived skin cell from over 100 people ages 1 to 94. By blending the expertise of molecular biology; machine-learning algorithms; and a technology called RNA-Seq, the team was able to identify the age of a person based on changes in expression across the cells’ genomes.
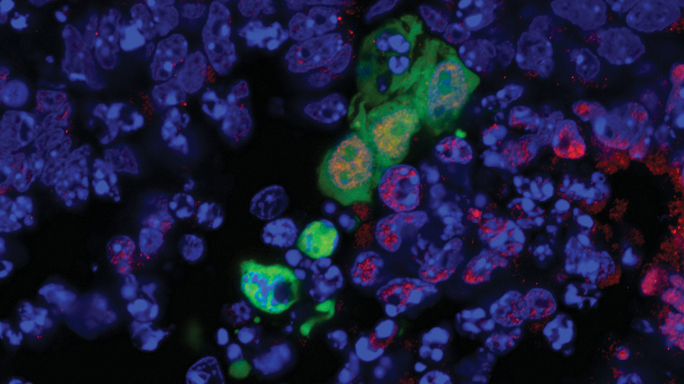
A human stem cell is shown in green integrating and developing into the surrounding cells of a mouse embryo. The new type of stem cell holds promise for one day growing replacement tissues.
Next, the researchers plan to look for biomarkers in other cell types and see whether this analysis could be used to predict age-related health conditions, determine aging changes during times of stress, and even develop targeted interventions.
In another approach to combating organ and tissue aging, Salk Professor Juan Carlos Izpisua Belmonte is developing stem-cell-based techniques to create therapies that can halt and, in some cases, even reverse signs of aging.
Stem cells have the ability to become virtually any cell in the body. After development, we retain limited reserves of specialized stem cells throughout adulthood to help replenish our tissues and organs, but scientists believe that harnessing the power of stem cells could lead to ways to repair or replace aging organs.
Izpisua Belmonte’s team starts with induced pluripotent stem cells–cells taken from an organ (skin, typically) and coaxed to revert back to a stem-cell-like state using a mixture of signaling proteins called Yamanaka factors. Since the discovery of Yamanaka factors, labs throughout the world have used induced pluripotent stem cells for research because, like embryonic stem cells, they are capable of dividing indefinitely and becoming almost any cell type.
But, as with telomerase, too much of a supposedly good thing can be bad. Attempts to introduce Yamanaka factors into cells inside the body to encourage them to take on stem-cell-like properties and regenerate tissue have failed–until recently.
In December 2016, the Izpisua Belmonte lab published a landmark study in Cell, showing for the first time that by exposing cells to Yamanaka factors for short periods of time, the researchers were able to keep cells stable while reversing age-associated hallmarks. In mice with Hutchinson-Gilford progeria syndrome (a genetic disorder also found in humans that causes accelerated aging), the results were striking: Compared with untreated mice, the reprogrammed mice looked younger; their cardiovascular function and the function of other organs improved; and, most surprisingly, the mice lived 30 percent longer yet did not develop cancer. Additionally, when this cyclic introduction of the Yamanaka factors was applied to normally aged mice, the team saw improved regenerative capacity of the pancreas and muscles.
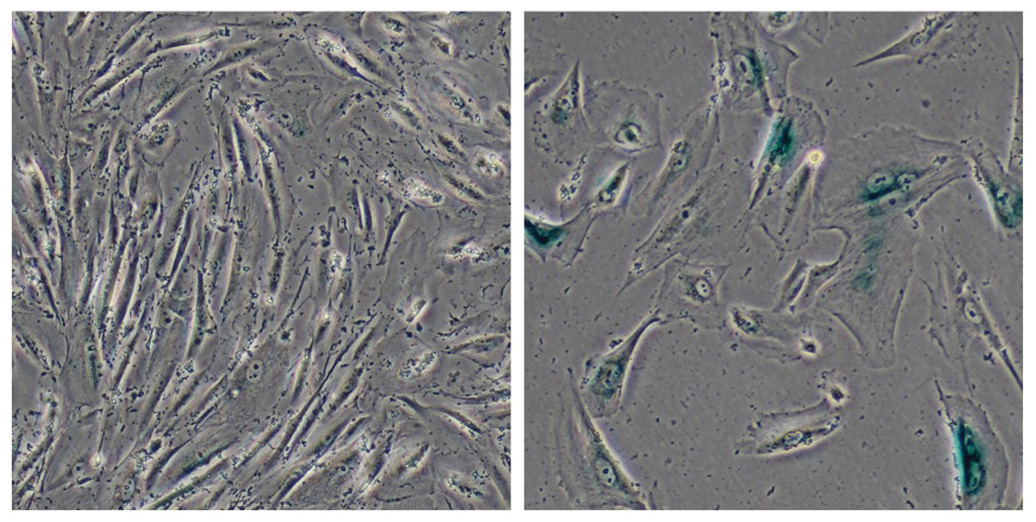
Salk scientists compare normal cells (left) to cells associated with a genetic disorder that leads to premature aging (right). The deterioration of bundles of DNA is seen in the prematurely aging cells, giving new clues to age-related disease.
“Our work showed that aging is a very dynamic and plastic process and therefore will be more amenable to therapeutic interventions than we previously thought,” Izpisua Belmonte says.
The lab is pursuing another solution for organ failure associated with aging and disease: Izpisua Belmonte’s 2017 Cell paper, which made international headlines, provided the first proof-of-concept study for how functional organs from one species can be grown in another–an early step in addressing the critical shortage of human donor organs available for transplant. His lab was successful in both growing rat organs in a developing mouse and in integrating human cells and tissues in early-stage pig and cattle embryos, marking the first step toward the generation of transplantable human organs using large animals whose organ size, physiology and anatomy are similar to humans’.
“As we continue to develop stem-cell models of human aging and aging-associated diseases and discover new drivers of aging, we are very optimistic for our work’s potential to extend lifespan and promote healthy aging,” Izpisua Belmonte says.
In addition to these stem-cell approaches, the lab is developing technologies related to genome and epigenome editing that allow for the activation of genes without creating breaks in DNA. In a study published in December 2017 in Cell, Izpisua Belmonte’s lab reported on a new technology that can be used to treat age-associated diseases that are caused by abnormal gene expression. And in a 2019 Nature Medicine paper, the lab used the CRISPR/Cas9 technology to prevent the accumulation of progerin, a toxic form of the lamin A protein, in mice with progeria.
“Age-related disease, particularly neurodegeneration, is one of the most pressing issues facing modern society … I believe we will start to see effective treatments emerge.”
-Rusty Gage

Saving the brain from cognitive decline and Alzheimer’s
Generating new pancreatic tissue is one thing, but how does one repair an aging or damaged brain?
Alzheimer’s disease, for example, represents a global health crisis with tens of millions of people afflicted, and the number of cases expected to grow. Aside from the disease being a public health burden worldwide, the erosion of one’s mind is terrifying and isolating for those afflicted and is devastating for families. Cognitive decline is one of the top fears of people over 50, according to a recent survey by AARP.
Currently, there is no cure for Alzheimer’s and other age-related dementias, but Salk scientists are working to change that.
Professor David Schubert, together with Senior Staff Scientist Pamela Maher, Staff Scientist Antonio Currais and colleagues, created a way to screen compounds from plants for their ability to protect and help recover damaged brain cells. With this technique, the lab can sift through thousands of molecules and pick out compounds for drugs to target neurodegenerative disease. The team can then home in on these molecules and, in some cases, make them more therapeutic by tweaking their chemical properties through medicinal chemistry.
Using this method, the Salk team has uncovered a handful of compounds that show promise for improving neural health and protecting against dementia, including fisetin, found in fruits and vegetables; curcumin from turmeric; and, most recently, a chemical called sterubin from a California plant called Yerba santa. These explorations are paying off: a curcumin derivative the team developed, called J147, is now in clinical trials for Alzheimer’s. J147 and the other drug candidates appear to reduce many of the toxicities that occur with aging and engage the same molecular pathways as several other anti-aging approaches (such as calorie restriction), according to Schubert.
“We have found a number of plant-derived molecules offer a range of protective properties, with many resulting in improved cognition in animal models of Alzheimer’s,” Schubert says. “These have the potential to be affordable and effective treatments for the disease, and, perhaps most importantly, they extend lifespan in model organisms and may delay or prevent the diseases of aging in humans.”
Salk research on Alzheimer’s continues to gain steam. In May 2018, the Institute received $1.5 million from the Korea-based company NANOS to establish the NANOS Alzheimer’s Disease Stem Cell Suite. This dedicated laboratory space enables Salk scientists to collect and store samples and data from a large number of individuals to more accurately pinpoint processes, like DNA repair, that go awry in Alzheimer’s disease.
Additionally, in November 2018, the American Heart Association-Allen Initiative in Brain Health and Cognitive Impairment awarded a team of 10 Salk labs $19.2 million over eight years to investigate mechanisms underlying age-related cognitive decline.
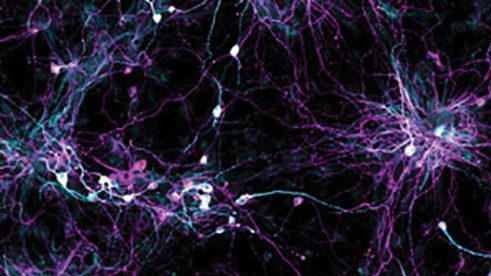
Salk scientists developed a technique to grow aged brain cells from patients’ skin (shown) while retaining cellular signatures of aging. The new technique will enable better studies of age-related disease.
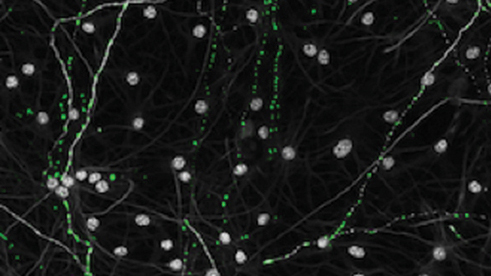
Salk researchers discovered that neurons (gray) from older individuals had impaired mitochondria (green) and reduced energy production, a finding that could reveal more about the link between mitochondrial dysfunction and age-related brain diseases.
This unprecedented, interdisciplinary effort, led by President Rusty Gage, approaches age-related cognitive decline as a failure of complex, interdependent biological networks in our bodies hat break down over time. These systems include metabolism, immunology and inflammation, genetics and epigenetics and protein interactions. By understanding why a breakdown in one of these systems causes a domino-like crash resulting in devastating dementia, the team aims to reveal new targets for therapeutic research and biomarkers of early-stage cognitive decline.
The Salk research teams are forging new tools and techniques to explore these interconnected networks and this cellular crosstalk. Novel cell cultures and brain organoids, a new primate model of cognitive aging, and state-of-the-art machine-learning algorithms will allow scientists to better understand how the brain ages.
“We are hopeful that, by looking into the role of inflammation and other cellular processes in Alzheimer’s, we can find breakthroughs humanity so desperately needs,” says Professor Susan Kaech, director of Salk’s NOMIS Center for Immunobiology and Microbial Pathogenesis, holder of the NOMIS Chair, and a member of this project’s leadership team.
In addition to Gage, Hetzer, Kaech, Karlseder, Navlakha and Shadel, other Salk investigators on the grant include Nicola Allen, an expert in supportive brain cells called astrocytes, Joseph Ecker, who has made groundbreaking discoveries in epigenetics, John Reynolds, who uses new models to chart the brain, and Reuben Shaw, who directs Salk’s Cancer Center and is an expert in cellular metabolism.
“Age-related disease, particularly neurodegeneration, is one of the most pressing issues facing modern society,” says Gage, who holds the Vi and John Adler Chair for Research on Age-Related Neurodegenerative Disease. “By exploring the effect of these biological systems on one another, as well as incorporating a strong computational piece to analyze all of the new data, I believe we will start to see effective treatments emerge.”
With the advent of new technologies, bold efforts and innovative collaborations, Salk scientists are optimistic that these–and many other–endeavors will lead to longer, healthier lives.
Support a legacy where cures begin.
Featured Stories
 Gerald Shadel explores stressed-out mitochondriaInside Salk sat down with Shadel to find out how he became interested in mitochondria, what he is driven by scientifically and what he has learned about aging along the way.
Gerald Shadel explores stressed-out mitochondriaInside Salk sat down with Shadel to find out how he became interested in mitochondria, what he is driven by scientifically and what he has learned about aging along the way. The science of agingWhat we know about longevity so far: Minimizing smoking, obesity and overeating while maximizing exercise and social interactions seem to correspond to longer and healthier lives, but not always. While many factors have been touted as panaceas for extending life–everything from adhering to a Mediterranean diet to regularly imbibing red wine–a “fountain of youth” has remained stubbornly elusive.
The science of agingWhat we know about longevity so far: Minimizing smoking, obesity and overeating while maximizing exercise and social interactions seem to correspond to longer and healthier lives, but not always. While many factors have been touted as panaceas for extending life–everything from adhering to a Mediterranean diet to regularly imbibing red wine–a “fountain of youth” has remained stubbornly elusive. Lillian Eichner: Reading the clues to fight cancerEichner began studying cancer while pursuing her PhD at McGill University in Montreal. She was drawn to the Salk Institute for her postdoctoral studies because Reuben Shaw, director of the Salk Cancer Center and a professor in Salk’s Molecular and Cell Biology Laboratory, has taken a new approach to cancer by studying the metabolic pathways of deadly tumors.
Lillian Eichner: Reading the clues to fight cancerEichner began studying cancer while pursuing her PhD at McGill University in Montreal. She was drawn to the Salk Institute for her postdoctoral studies because Reuben Shaw, director of the Salk Cancer Center and a professor in Salk’s Molecular and Cell Biology Laboratory, has taken a new approach to cancer by studying the metabolic pathways of deadly tumors.