FRONTIERS How to Stop a Killer New approaches to pancreatic cancer research
Dannielle Engle has a deeply personal interest in finding a way to diagnose pancreatic cancer early: Her father and her uncle died of it. Now an assistant professor in Salk’s Regulatory Biology Laboratory, Engle was an undergraduate at Northwestern University when her father was diagnosed with pancreatic cancer 15 years ago. For a little while, his treatment—surgery and then a two-drug chemotherapy regimen—seemed to have been successful. But the cancer returned, and this time it didn’t go away.
Engle, who was very close to her father, says he was one of the lucky ones: He lived for 14 months after his initial symptoms became apparent, versus the typical 4-6 months following diagnosis.
This year, around 57,000 people are expected to be diagnosed with pancreatic cancer and 46,000 will die from it, according to the American Cancer Society, making it one of the deadliest cancers.
Part of the problem in treating pancreatic cancer is that it’s hard to detect. In the case of Engle’s father, the cancer had been growing quietly for five years before it was diagnosed. One reason for a late diagnosis is that many of the early symptoms of pancreatic cancer—digestive issues, back pain, jaundice—are associated with numerous other health problems, including diabetes and a condition called pancreatitis, in which the pancreas is inflamed.
Another reason this cancer is so hard to defeat is that the pancreas, a smartphone-sized abdominal gland that releases digestive juices into the intestine and releases hormones (such as insulin) into the bloodstream, is difficult to image due to its location in the body.
Once pancreatic cancer is diagnosed, surgery is an option, but only if the cancer has not metastasized.
That’s why the Salk Institute has made pancreatic cancer a focus in its Conquering Cancer Initiative, a dedicated research effort that was launched in spring 2018 to tackle five of the deadliest cancers. In addition to pancreatic cancer, Salk faculty are using a variety of research innovations to reveal insights into brain, lung, ovarian and triple-negative breast cancers.
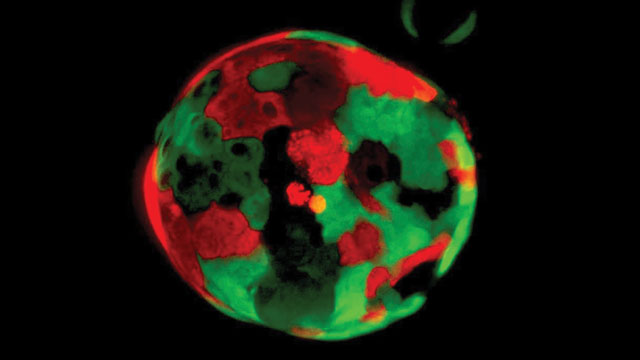
Pancreatic organoids
Organoids are simplified, miniature versions of organs, used for research and, in particular, to model disease (see this issue’s “Analysis” to learn more about organoids).
Engle is building a repository of pancreatic organoids, derived from both healthy and cancerous tissue, to enable her team to identify differences between the two tissue types—especially at different stages of cancer development—that could be used for diagnostic or therapeutic purposes.
The Engle lab has more than 100 self-renewing pancreatic cancer organoids, most of which are grown from tumor biopsy tissue, which means they have the exact genetic profile of the cancer patient from whom they are derived. Understanding genetic variations in tumors could be a powerful tool in advancing treatments and a cure for the disease.
Image credit: Cold Spring Harbor Laboratory/Tuveson Lab/Dannielle Engle
The effort harnesses Salk’s National Cancer Institute-designated Cancer Center in the hope that the approaches—ranging from new models of treatment to unexpected collaborations across disciplines and international boundaries—will facilitate better treatments not just for these cancers but for all cancers.
On May 28, 2019, Salk’s Cancer Center launched a bold effort in which Salk labs are facilitating international translational alliances to seek a cure for pancreatic cancer. Salk labs are already making headway, as milestone discoveries have appeared in numerous journals and led to four trials using novel approaches.
“At Salk, our goal is to push boundaries and come up with ideas that other places don’t envision,” says Reuben Shaw, the director of the Salk Cancer Center and the William R. Brody Chair. “Simply said, innovation and insight let us go where others have not gone.”
“We take on the toughest challenges because those breakthroughs lead to so much more,” says Ronald Evans, director of Salk’s Gene Expression Laboratory. “Beyond the lab bench, Salk has built a network of clinical collaborators, enabling us to transform discovery into treatment. We’re at an inflection point for pancreatic cancer research, in which momentum and pace are moving faster than ever before, leading to innovative treatments even for the most advanced forms of the disease.”
Finding a needle in a haystack
Watching her father deal with pancreatic cancer made Engle want to work on developing methods of early detection for the disease. As a graduate student, she conducted research in the lab of Salk faculty member Geoffrey Wahl. Now, as head of her own lab, she develops pancreatic cancer organoids, which can be grown from a sliver of tumor the thickness of a human hair.
These organoids may well be game changers for pancreatic cancer research. Engle is using organoids to test diagnostic methods and treatments. (See this issue’s “Analysis” to learn more about organoids.)
One of Engle’s diagnostic approaches focuses on a carbohydrate called CA19-9. Levels of this sugar are elevated in the blood of pancreatic cancer patients. When people are responding to therapy, the levels decrease, making CA19-9 a valuable indicator of therapeutic effectiveness. Unfortunately, levels are also elevated in pancreatitis, and telling the two conditions apart is difficult. Cancer researchers, Engle among them, think that CA19-9 could be a useful biomarker for early pancreatic cancer detection, if the sugar could be better understood.
“Looking for biomarkers is the age-old problem of looking for a needle in a haystack,” Engle says. “The blood contains signals not just from the pancreas but from every tissue in the body. So 99.9 percent of your time as a researcher is spent trying to exclude signals that are not relevant. In other words, you spend most of your time in the hay.”
Engle is seeking to change that. In June, she published a groundbreaking paper in the journal Science, in which she discussed using organoids and mouse models to reveal that CA19-9 is not merely correlated with pancreatitis and pancreatic cancer but can actually contribute to both conditions. Her research suggests that the sugar has the potential to act as either an accelerator or a brake for both cancer genes and tumor-suppressor genes. In addition, she showed that using antibodies to block CA19-9 reversed pancreatitis and made pancreatic cancer less aggressive, which could buy patients valuable time, during which other therapies may work.
Engle is working to develop a large collection of organoids derived both from healthy pancreata and from inflamed or cancerous pancreata in order to compare the types and come up with biomarker candidates for use in developing diagnostic blood tests.
Her lab will also be able to use the organoids for drug testing and analysis of tumors’ genetic vulnerabilities.

Breaking down the cancer’s shield
Evans, the Gene Expression Laboratory director who talked about being at an inflection point in pancreatic cancer research, also has a familial connection to cancer: His father died of glioblastoma, his brother died of leukemia, and his sister died of kidney cancer.
Initially, Evans, who also holds the March of Dimes Chair in Molecular and Developmental Biology, didn’t set out to work on cancer at all, much less pancreatic cancer. The Salk professor and Howard Hughes Medical Institute investigator is an authority on hormones, molecules that carry messages throughout the body. He is known for discovering a large family of molecules called nuclear receptors, some of which respond to steroid hormones, such as vitamins A and D.
In 2013, Evans’ lab was profiling hundreds of cell types to map the location of every nuclear hormone receptor and to perhaps yield something about their function. The team noticed that, unexpectedly, there were very high levels of vitamin D receptors in liver stellate cells. Vitamin D receptors act as sensors for organ injury and respond by telling other liver cells to produce fibrous proteins (similar to scar tissue) to heal the wound. Evans wondered why there were so many vitamin D receptors in stellate cells.
The team’s follow-up experiment revealed an answer: The receptor acts as a molecular “on-off” switch, controlling liver fibrosis in a hormone-dependent fashion. Without vitamin D, inflammation and scarring are relentless. With vitamin D, inflammation and scarring resolve and the wound heals. The 2013 study, which was published in the journal Cell, was exciting not only for its implications for liver fibrosis and liver cancer—but because the pancreas also has stellate cells, suggesting an unexpected role for the vitamin D receptor in the development and treatment of pancreatic cancer.
It turns out that the pancreas treats cancer cells as an injury needing to be healed, so it sounds the inflammatory alarm, summoning various immune cells and fibroblasts that trigger the fibrotic response. Together, these cellular components form an impenetrable tangle—the stroma—which, ironically, functions as a shield for the tumor, hiding and protecting it from immune cells and therapeutic drugs. This wall of scar tissue surrounding the tumor is one of the main reasons pancreatic cancer is so difficult to treat.
Described in 2014, again in the journal Cell, Evans’ lab found that inflamed pancreatic stellate cells had higher levels of vitamin D receptors than did normal pancreatic stellate cells. When the researchers gave synthetic vitamin D to mice that had inflamed pancreases, the vitamin again appeared to act as a switch, calming the cells’ inflammatory signaling and allowing them to return to a normal, uninflamed state. Given those promising results, the team was eager to try their vitamin D therapy in the tumor environment.
They found that administering the vitamin D analog along with chemotherapy in a mouse model of pancreatic cancer enabled those mice to live 50 percent longer than mice receiving only chemotherapy. The reason was dramatic: In calming the inflammation, the vitamin D analog opened up the normally impenetrable fibrotic shell, allowing chemotherapy drugs and tumor-seeking immune T cells to find and attack the tumor.
The vitamin D receptor is what Evans refers to as a “master regulator,” controlling about 250 genes involved in cellular communication, immunity and other functions. But pancreatic tumors degrade vitamin D, essentially making tumors into wounds that cannot heal. However, the chemically engineered form of vitamin D that Evans uses enables it to penetrate the tumor environment without becoming degraded.
“Vitamin D is literally a hormone activated by light,” says Evans. “So, when cancer prevents it from functioning, the tumor is able to stay hidden and in the dark, figuratively speaking. That’s where our vitamin D analog therapy comes in, acting like liquid light. It flips all the cellular switches the cancer had reprogrammed and sheds new light on the disease, so to speak. The tumor begins to regress, creating vulnerabilities that drugs or the immune system can exploit.”
Salk pancreatic cancer research led by Evans has clinical-trial alliances with Dana-Farber Cancer Institute, Memorial Sloan Kettering Cancer Center, City of Hope, UC San Diego’s Moores Cancer Center, the University of Pennsylvania and others. In fact, Evans’ vitamin D analog is currently in human clinical trials, and one patient, Stephen Bigelsen, believes it saved his life. The New York City-based physician was diagnosed with stage IV metastatic cancer in 2016. Surgery wasn’t even an option. Bigelsen and his wife, Susan, heard about the experimental vitamin D therapy from his medical team at Weil Cornell Medical Center and decided to try it. About one month into the combination treatment of vitamin D plus chemotherapy, when his first blood tests showed improvement, he began to have hope. Within a year, his cancer was completely gone. Today, Bigelsen serves as a pancreatic cancer patient advocate, educating others about the disease.
“Though it is one case, and more research needs to be done, it is dramatic and proves that new and effective therapies can make their way from the Salk bench to the patient’s bedside,” Evans says. “Staying ahead of the curve, we already have two more discoveries that are on their way to the clinic. This reinforces the idea that ‘knowledge is power,’ as well as that pancreatic cancer is treatable.”
“Staying ahead of the curve, we already have two more discoveries that are on their way to the clinic. This reinforces the idea that ‘knowledge is power,’ as well as that pancreatic cancer is treatable.” – Ronald Evans
Disrupting cancerous communication
American Cancer Society Professor Tony Hunter also has made progress in dismantling the wall around pancreatic tumors by focusing on the communication between tumor cells and stellate cells.
Researchers already knew that noncancerous cells are helping to create the pancreatic stroma, but they weren’t sure how.
“A tumor is basically a new type of tissue, and in every tissue, cells talk to one another,” says Hunter, who holds the Renato Dulbecco Chair at Salk. “We wanted to understand what they were saying.”
Hunter’s team began by using cell cultures to analyze all of the proteins that stellate cells were exporting, and the team found that inflamed pancreatic stellate cells were producing a protein called LIF (short for leukemia inhibitory factor). LIF was already known for its role in helping embryonic stem cells maintain their full developmental potential, but LIF is usually not present in adults. What, then, was it doing in adult pancreatic stellate cells?
Further experiments in a mouse pancreatic cancer model revealed that LIF was helping tumor cells grow. When LIF was blocked by administering an antibody, tumor growth slowed, giving chemotherapy drugs a chance to work. Equally exciting was the lab’s finding that LIF levels in patients were significantly correlated with pancreatic tumor progression and response to chemotherapy. This suggests that LIF, like CA19-9, holds promise as a biomarker for pancreatic cancer stage and treatment response.
The team published their findings in the journal Nature in April 2019, and a clinical trial testing an antibody against LIF is now being conducted by a biotech company in Toronto, in partnership with Celgene Corporation and Stand Up To Cancer.
Understanding how cancer begins
Professor Geoffrey Wahl, deputy director of the Salk Cancer Center, likes to quote pathologist Harold Dvorak, who referred to cancer as “the ever-healing wound that never heals.” Wahl aims to identify what the wound-healing responses in cancers have in common. He studies how cancers begin, and how they acquire the complexity that contributes to both drug resistance and metastasis.
He and Staff Scientist Kathleen DelGiorno (a co-author on Hunter’s LIF paper), are investigating how chemical-sensing cells called tuft cells are involved in pancreatic cancer progression. Wahl and DelGiorno made the surprising finding that tuft cells are not present in the normal pancreas, but arise in response to injury or mutations to the KRAS gene that are found in 90 percent of human pancreatic cancers. DelGiorno discovered that tuft cells can actually quell pancreatic inflammation to help the inflamed organ heal, but in the context of cancer-causing mutations, the growing cancer somehow leads to tuft cell disappearance. In the absence of tuft cells, the cancer grows more aggressively. The team now knows how tuft cells mitigate cancer progression, and hope to turn these new findings into therapies to ease pancreatitis and possibly to slow pancreas cancer progression. A second team member, Postdoctoral Fellow Nikki Lytle, studies how tissue perturbations associated with obesity and other conditions can promote pancreatic tumor initiation or progression to metastasis, particularly in high-risk patient populations.
Wahl, who holds the Daniel and Martina Lewis Chair, comments that one underlying principle in all deadly cancers is the cellular plasticity, or flexibility, that leads to the development of different types of cells within the primary cancer and its metastases.
“The intratumoral cellular heterogeneity is the imprecision of precision medicine,” says Wahl. “To defeat the most deadly cancers, we must find and treat them earlier, and develop new approaches to prevent the tumors from acquiring such complexity.”
This remains one of the great challenges of cancer biology, but Wahl is confident in the ability of the highly collaborative teams within the Salk Cancer Center to succeed.
Tapping the body’s own defenses
The Evans and Hunter labs’ approaches to breaching the stroma allow chemotherapy drugs to access tumors, but Salk Professor and NOMIS Chair Susan Kaech is hoping to make a person’s own immune system do the work of chemotherapy, without the toxicity of those types of drugs, as part of a rapidly evolving field known as cancer immunotherapy.
“Immunotherapy is a new form of cancer treatment that is revolutionizing the way we’re using drugs. The goal is to stimulate our immune system to identify and defeat cancer cells in a ‘seek and destroy’ way,” says Kaech, director of Salk’s NOMIS Center for Immunobiology and Microbial Pathogenesis.
Immune T cells are activated when we encounter a pathogen, like bacteria or a virus, forming a molecular memory of it so that the next time we’re exposed, our bodies can quickly mount an efficient response. Even though cancer is not a foreign pathogen, cancer mutations can make otherwise normal cells look foreign to our immune system, allowing T cells to recognize and attack them. Unfortunately, pancreatic cancer doesn’t have as many mutations as other cancers, which means it’s harder for our immune system to recognize. In addition, the impenetrable barrier of the stroma prevents even immune cells that recognize the tumor from accessing it.
Kaech is working to change that. She discovered that tumors cause immune suppression, in part, by quashing the metabolism of T cells. Just as people aren’t as productive if they’re hungry, T cells can’t function properly if a tumor is hogging all of the available nutrients. Her work suggests that efforts to starve tumors may actually hurt immunity in the process because of the way tumors and immune cells influence each other metabolically.
Kaech is also working to identify how other types of immune cells might be recruited to fight cancer. Although T cells can’t penetrate the stroma, immune cells called macrophages can. But macrophages—cells that “eat” other cells by breaking them down—can also make the tumor environment immunosuppressive. By better understanding how various immune cells are regulated, Kaech aims to improve immunotherapy techniques.
Tackling cancer from all angles
If cancers have underlying commonalities, so do Salk’s cancer researchers. Salk’s collaborative model, which brings together researchers from a variety of biological backgrounds, may be the best way to make progress in the search for effective therapies not only for pancreatic cancer but for all of the hardest-to-treat cancers.
“Salk attracts scientists who have big, bold ideas and want to make a difference,” Engle says. “It doesn’t matter how wild your idea is. You can make it happen at Salk, even if that means reinventing who you are as a scientist or adopting a new approach that nobody’s ever tried before. This is one of the only places in the world where you can do that.”
As part of the Conquering Cancer Initiative, the pancreatic cancer research team has taken the first step to expand the program into an international translational alliance that will allow discoveries from Salk to have worldwide impact.
“What we learn about pancreatic cancer gives us powerful tools to help fight other deadly cancers,” Evans says. “We’re a small institute, but our goal is to tackle big problems. The harder they are, the bigger the reward when you find a solution. And I feel optimistic that with pancreatic cancer we will find solutions, with a goal for the cure.
Support a legacy where cures begin.
Featured Stories
 Kay Tye – Breaking down the brainKay Tye, the newest addition to Salk’s faculty, is a burst of energy who can chat about everything from the mysteries of the brain to the intricacies of a breakdance move. In this Q&A, she discusses her roundabout journey to science, her passion for mentorship and her love of life outside the lab.
Kay Tye – Breaking down the brainKay Tye, the newest addition to Salk’s faculty, is a burst of energy who can chat about everything from the mysteries of the brain to the intricacies of a breakdance move. In this Q&A, she discusses her roundabout journey to science, her passion for mentorship and her love of life outside the lab. How to stop a killerPancreatic cancer is one of the most difficult cancers to detect and treat, in part because of an impenetrable "shield" that forms around the tumor. Salk scientists, many of whom have a personal connection to cancer, are leading the charge in new approaches to tackle this deadly disease.
How to stop a killerPancreatic cancer is one of the most difficult cancers to detect and treat, in part because of an impenetrable "shield" that forms around the tumor. Salk scientists, many of whom have a personal connection to cancer, are leading the charge in new approaches to tackle this deadly disease. Travis Berggren – Working at the intersection of biology and technologySenior Staff Scientist Travis Berggren shares his path to Salk and his perspective on how advances in technology facilitate world-changing discoveries in genetics, neuroscience, cancer, immunology, plant biology and other areas.
Travis Berggren – Working at the intersection of biology and technologySenior Staff Scientist Travis Berggren shares his path to Salk and his perspective on how advances in technology facilitate world-changing discoveries in genetics, neuroscience, cancer, immunology, plant biology and other areas.





















































