Kaech, director of the NOMIS Center for Immunobiology and Microbial Pathogenesis, has been elected to the American Academy of Arts and Sciences. She shares the honor with some of the world’s most accomplished leaders from science and technology, business, public affairs, education, the humanities, and the arts. Kaech and the new class of nearly 270 members will be inducted at a formal ceremony on September 30, 2023, at the Academy’s headquarters in Cambridge, Massachusetts.
Kaech, who holds the NOMIS Chair, is an immunologist who has contributed significantly to the understanding of how long-term immunity forms. Specifically, she helped establish the modern understanding of how and when memory T cells form. Memory T cells are critical for maintaining long-term immunity during acute and chronic infections and can be suppressed in cancer. Kaech identified several genes and signaling molecules critical for memory T cell generation during immune responses. She also helped establish the field of cancer immunometabolism by discovering that the metabolic interplay between tumor and immune cells, as well as changes in nutrient availability, can lead to metabolic immune suppression in tumors. Her findings have solidified the framework for understanding how memory T cells form during infection and may lead to new therapeutic interventions for cancer in the future.
Kaech is also a Fellow of the American Association for the Advancement of Science and has received numerous awards, including a Howard Hughes Medical Institute Early Career Scientist award, the National Institutes of Health Presidential Early Career Award for Scientists and Engineers, the Burroughs-Wellcome Fund Career Award in the Biosciences, and the Damon Runyon-Walter Winchell Cancer Research Fellowship, among others. She was also recently elected to the American Association of Immunologists Council, which serves as the largest and most influential organization to advance the knowledge of immunology and medical research in the country.






























































 Dmitry Lyumkis, holder of the Hearst Foundation Developmental Chair, investigates the mechanisms by which biological invaders (pathogens), like viruses, interplay with their hosts to establish and maintain infection. His lab uses multidisciplinary biophysical techniques centered on cryo-electron microscopy to understand how viral and host proteins assemble, interact, and produce diverse functional outcomes. Understanding the form and function of proteins helps unravel the complex roles they play in viral infections and in human diseases, such as cancer, and informs therapeutic strategies to target those diseases. He recently determined the molecular structure of HIV Pol, a protein that plays a key role in the late stages of HIV replication when the virus begins spreading throughout the body.
Dmitry Lyumkis, holder of the Hearst Foundation Developmental Chair, investigates the mechanisms by which biological invaders (pathogens), like viruses, interplay with their hosts to establish and maintain infection. His lab uses multidisciplinary biophysical techniques centered on cryo-electron microscopy to understand how viral and host proteins assemble, interact, and produce diverse functional outcomes. Understanding the form and function of proteins helps unravel the complex roles they play in viral infections and in human diseases, such as cancer, and informs therapeutic strategies to target those diseases. He recently determined the molecular structure of HIV Pol, a protein that plays a key role in the late stages of HIV replication when the virus begins spreading throughout the body. Sung Han, holder of the Pioneer Fund Developmental Chair, works to understand how the brain recognizes environmental threats and sends signals that change physiology, metabolism, behavior, and emotion to avoid those threats. When this threat signaling pathway goes awry, it can create hypersensitivity, a characteristic of neuropsychiatric disorders, such as post-traumatic stress disorder (PTSD), as well as other panic and anxiety disorders. He recently uncovered a molecular pathway that initiates a fear response and described the connection between feelings of fear and breathing rhythm. Han also works to understand the science behind overdoses, which led to his discovery of a group of neurons that play a key role in the disrupted breathing that is often characteristic of overdose deaths.
Sung Han, holder of the Pioneer Fund Developmental Chair, works to understand how the brain recognizes environmental threats and sends signals that change physiology, metabolism, behavior, and emotion to avoid those threats. When this threat signaling pathway goes awry, it can create hypersensitivity, a characteristic of neuropsychiatric disorders, such as post-traumatic stress disorder (PTSD), as well as other panic and anxiety disorders. He recently uncovered a molecular pathway that initiates a fear response and described the connection between feelings of fear and breathing rhythm. Han also works to understand the science behind overdoses, which led to his discovery of a group of neurons that play a key role in the disrupted breathing that is often characteristic of overdose deaths. Graham McVicker, holder of the Frederick B. Rentschler Developmental Chair, studies how human genetic differences, known as genetic variants, affect traits and diseases. In the past decade, thousands of genetic variants have been associated with human diseases. However, the function of most of these variants is unknown and difficult to determine, since they are often in what are called “noncoding” portions of the human genome. Noncoding regions do not provide instructions for making proteins that enable cell function. Instead, research suggests they influence when and where (under which conditions and in which cell types) specific proteins are made by the “coding” portions of the genome. McVicker uses CRISPR and computational analysis to understand the function of each genetic variant in every cell type, with a particular focus on understanding cancer and diseases related to the immune system. His long-term goal is to reveal novel disease mechanisms that could support the development of personalized therapies. He was recently awarded a Curebound Discovery Grant and a Genomic Innovator Award.
Graham McVicker, holder of the Frederick B. Rentschler Developmental Chair, studies how human genetic differences, known as genetic variants, affect traits and diseases. In the past decade, thousands of genetic variants have been associated with human diseases. However, the function of most of these variants is unknown and difficult to determine, since they are often in what are called “noncoding” portions of the human genome. Noncoding regions do not provide instructions for making proteins that enable cell function. Instead, research suggests they influence when and where (under which conditions and in which cell types) specific proteins are made by the “coding” portions of the genome. McVicker uses CRISPR and computational analysis to understand the function of each genetic variant in every cell type, with a particular focus on understanding cancer and diseases related to the immune system. His long-term goal is to reveal novel disease mechanisms that could support the development of personalized therapies. He was recently awarded a Curebound Discovery Grant and a Genomic Innovator Award.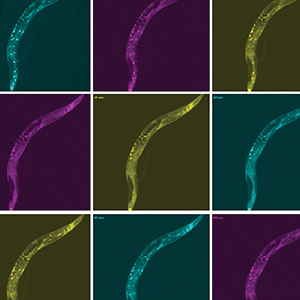 Sreekanth Chalasani studies how animals make complex strategic decisions, such as balancing the quest for food with territorial defense and predator avoidance. His work has revealed the brain circuits that underlie these complex decisions. He also uses worm and mouse models to pinpoint the differences between healthy and dysfunctional brains, make inferences about the human brain, and to shed light on conditions like autism spectrum disorder, post-traumatic stress disorder (PTSD), anxiety, and depression. He recently discovered how hunger signals in the gut communicate with the brain, and identified the cells and connections in the brain that facilitate decision-making. Additionally, he engineered mammalian cells to be activated by sonogenetics, paving the way for other noninvasive versions of deep brain stimulation, pacemakers, and insulin pumps.
Sreekanth Chalasani studies how animals make complex strategic decisions, such as balancing the quest for food with territorial defense and predator avoidance. His work has revealed the brain circuits that underlie these complex decisions. He also uses worm and mouse models to pinpoint the differences between healthy and dysfunctional brains, make inferences about the human brain, and to shed light on conditions like autism spectrum disorder, post-traumatic stress disorder (PTSD), anxiety, and depression. He recently discovered how hunger signals in the gut communicate with the brain, and identified the cells and connections in the brain that facilitate decision-making. Additionally, he engineered mammalian cells to be activated by sonogenetics, paving the way for other noninvasive versions of deep brain stimulation, pacemakers, and insulin pumps.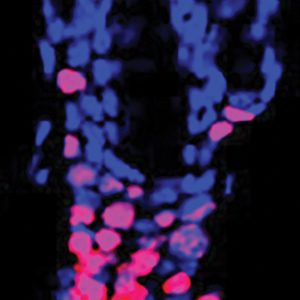 Ye Zheng studies the immune system dysfunction that causes inflammation and autoimmune disorders like rheumatoid arthritis, multiple sclerosis, type 1 diabetes, and asthma. Zheng focuses on regulatory T cells, which control immune responses and whose dysfunction has been linked to multiple autoimmune diseases. By looking at the genes that control regulatory T cells, he hopes to find new ways to manage T cell dysfunction and inspire future therapeutics. Recently, Zheng found a new target for alopecia treatment by discovering that regulatory T cells communicate with hair follicles to enable hair regeneration. Additionally, when looking at allergic skin inflammation in mice, he found that obesity changes the molecular underpinnings of allergic reactions—a finding that may have implications for allergies in humans too.
Ye Zheng studies the immune system dysfunction that causes inflammation and autoimmune disorders like rheumatoid arthritis, multiple sclerosis, type 1 diabetes, and asthma. Zheng focuses on regulatory T cells, which control immune responses and whose dysfunction has been linked to multiple autoimmune diseases. By looking at the genes that control regulatory T cells, he hopes to find new ways to manage T cell dysfunction and inspire future therapeutics. Recently, Zheng found a new target for alopecia treatment by discovering that regulatory T cells communicate with hair follicles to enable hair regeneration. Additionally, when looking at allergic skin inflammation in mice, he found that obesity changes the molecular underpinnings of allergic reactions—a finding that may have implications for allergies in humans too.






