In 1979, Professor Tony Hunter had only recently started his lab at Salk when he saw something no one else had even thought to look for before—a cellular switch called tyrosine phosphorylation. Today, more than 60 cancer drugs work because they inhibit that switch.
To survive and replicate, viruses hijack their host’s cell machinery, turning them into mini virus-making factories. It’s in a virus’ best interest to force its host into making more cells. But it’s bad news for the host when those cells start dividing out of control, and eventually become tumors.
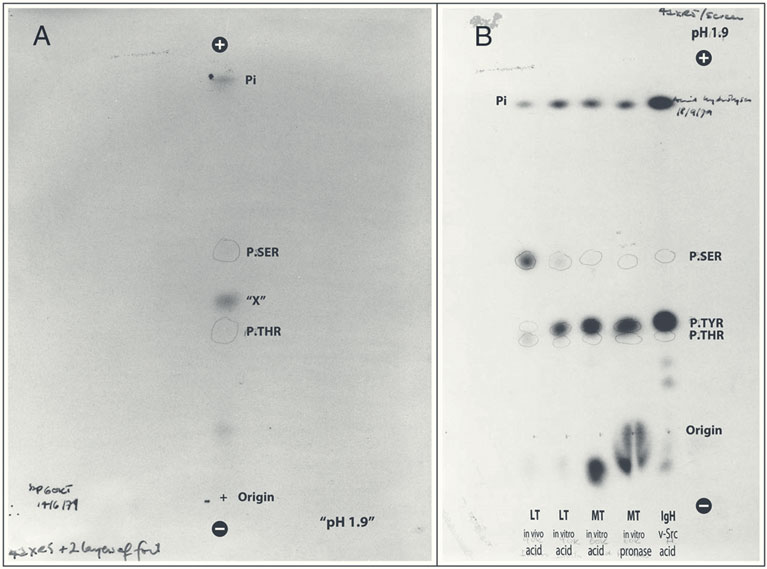
In the 1970s, excitement was growing about tumor viruses—viruses that cause cancer in animals. Hunter, then a junior faculty member at Salk, Karen Beemon, a member of his lab, and Bart Sefton, another junior faculty member, were studying two such viruses: polyomavirus and Rous sarcoma virus, which cause cancer in mice and chickens, respectively.
“Because these viruses are so simple—one has just four genes and the other has six—we hoped we could figure something out about how one or a few genes are able to convert normal cells into cancer,” says Hunter, who, turning 80 this year, is now Salk’s most senior faculty member, American Cancer Society Professor, and holder of the Renato Dulbecco Chair.
Not only would that information help them understand virally induced tumors in animals, Hunter thought, but it might also illuminate the ways human cells become cancerous.