Frontiers Human Connection How social interaction and isolation influence our physical and mental health
If there’s one thing the COVID-19 pandemic made abundantly clear, it’s that staying healthy involved more than merely avoiding the coronavirus itself. For many people, the social disruption and isolation wrought by the pandemic was emotionally challenging. According to a March 2022 scientific brief from the World Health Organization (WHO), anxiety and depression increased by 25 percent worldwide during the pandemic’s first year.
As a social species, humans need connection and interaction to thrive. Studies are increasingly linking social isolation and loneliness to a variety of negative health outcomes. These include physical conditions like heart disease and strokes, psychiatric issues like anxiety and depression, and cognitive problems like dementia.
Salk neuroscientists study how our brains allow us to experience, interpret, and interact with the world around us. They explore how individual brain cells and chemicals help us pursue rewards and avoid harm. Many of these researchers are now studying not only the brain alone, but the brain in a social context.


Even before the pandemic, Salk Professor Kay Tye, a Howard Hughes Medical Institute investigator and the Wylie Vale Chair, was investigating the neural circuitry of social behavior and the emotions behind it.
In 2016, her lab (then at MIT) identified a cluster of neurons in mice that were associated with a drive to socialize after being isolated from other mice. The team thought these neurons—part of a group of neurons called the DRN in the brainstem—might respond to what humans refer to as loneliness.
In mice housed together, DRN neurons were not very active.
“But after a mouse was separated from the group and reintroduced after time alone, its DRN neurons went wild,” Tye says. “In fact, the reintroduced mouse was even more social than the others, suggesting a rebound effect from being isolated.”
Mice whose DRN neuron activity was purposely suppressed didn’t experience this surge in sociability after isolation, supporting the idea that the DRN “loneliness” neurons were driving the urge to interact.
In 2020, Tye (by then at Salk) and her MIT colleagues published the results of a follow-up study in humans. This study focused on a small brain region called the substantia nigra, which has been implicated in people’s cravings for drugs. It is thought to be evolutionarily related to the DRN neurons in mice.
Healthy volunteers were required to have one session in which they fasted for 10 hours at home, and another in which they were socially isolated in a windowless room for 10 hours. Every two hours, the subjects completed questionnaires about how they were feeling, physically and emotionally.
In the social-isolation session, subjects were allowed to read preapproved documents, work on puzzles, or play solitary games (such as Tetris), but they were not allowed any social contact, including on their phones. Meals were dropped off outside the door to the room.
After each type of session, subjects had their brain activity measured by functional MRI (fMRI) while viewing stock photos—either of yummy-looking food (after the fasting session) or of people enjoying social interactions (after the isolation session). A baseline fMRI of each person was done at the beginning of the study.
The post-session fMRIs showed similar brain activity in the substantia nigra following both fasting and social isolation.
“This suggested to us that we crave social contact when we are lonely the way we crave food when we are hungry,” Tye says.

“This suggested to us that we crave social contact when we are lonely the way we crave food when we are hungry.”
– Sung Han
What’s more, the level of brain activity was correlated with people’s questionnaire ratings of how strongly they craved either food or social activity. So people who felt hungrier or lonelier had stronger brain activity when viewing images of food or socializing than people who felt less hungry or lonely.
Together, these studies point to specific brain circuits that drive mice and humans to seek social interaction, a process that can be impaired in anxiety disorders, depression, and addiction, among other neuropsychiatric conditions.
“Alcohol sales went up something like 20 percent in 2020,” says Tye. “It’s not how everyone coped with stress and loneliness, but certainly epidemiological data suggests a significant number of people did.”
(In fact, Tye lab postdoctoral researcher Reesha Patel—soon joining the faculty at Northwestern University—is nearing publication of a study of how social isolation affects alcohol consumption.)
Social animals naturally form hierarchies, with varying degrees of status among the members. Tye speculates that if you’re used to being top dog (or top mouse), you might feel lonely sooner than a mouse who’s getting bullied by other mice every day. Indeed, her graduate student Christopher Lee—while investigating how social isolation affects animals over time—is finding that dominant animals make distress calls sooner after being isolated than subordinate animals do.
To tackle not only a deficit in the quantity of social contact but also in the quality, the team wanted to explore social exclusion. Tye and graduate student Caroline Jia are studying social exclusion via more clever experiments with mice. In one study, a mouse is given treats but separated from its cage-mates by glass. While neural activity is recorded, the mouse can see the others also getting treats, but can’t join them. Mice that are excluded show increased sensitivity to physical pain, suggesting there is a common neurological basis for physical and social pain.
The results are not in for Lee’s and Jia’s experiments yet, but for all Tye’s research, the point is to illuminate the neural circuitry of social behavior, both to reduce stigma about mental illness and to find better treatments for it.
“When we think about the deficits of social contact, there’s quantity, and then there’s quality,” says Tye. “The pandemic showed us the effects of both. Maybe you had almost no social contact at all, and that was terrible. Or you got COVID and had to isolate temporarily and so you had Thanksgiving dinner by Zoom, while the rest of the family was all together. And that was rough, too. The pandemic really brought it all to the forefront.”


While Tye is deciphering the neural circuitry of loneliness, Associate Professor Kenta Asahina is investigating the underpinnings of aggression. In 2014, he discovered a brain chemical—a neuropeptide—responsible for aggressive behavior in male flies. A mammalian version of this chemical has also been linked to aggression.
More recently, Asahina’s lab has been looking at the effects of social contact in flies. Male flies who aren’t reared together are typically aggressive toward each other when meeting for the first time. In a study published in September 2022, the team describes how a gene called nervy is responsible for reducing aggression in male flies after they get used to each other’s presence.
The effect is more complicated than dialing aggression up or down by turning nervy “on” or “off,” as other genes are involved. But what’s important to understand, Asahina says, is that “this work suggests there are dedicated circuits in the animal brain that are sensitive to social experience or social isolation.”
This made Asahina wonder whether social isolation impacts life span differently in male and female flies. In most species, females live longer than males, but the versatility of flies as genetic models allowed Asahina to examine the question in a unique way: Because fly neurons are determined by sex, it’s possible to genetically engineer flies with female brains and male bodies, or female bodies and male brains. The lab tested the effects of social isolation on both types of engineered flies as well as fully female and fully male flies.
As expected, fully female flies (female body, female brain) lived the longest. But in the other groups, “things got more interesting,” Asahina says.
Flies with male brains didn’t live as long. Something about the male brain appears to shorten life span. Social isolation, however, did not seem to significantly impact life span in either sex. “Male-specific motivations or behaviors, such as fighting, may be more costly,” says Asahina.
In work that is currently underway, Donovan Ventimiglia, a postdoctoral researcher in Asahina’s lab, has found that male flies that are beaten up repeatedly by other males lose their motivation to fight back and begin trying to get away.
In both flies and humans, the neurochemical dopamine plays an important role in behavior related to motivation and reward-seeking.
But when Ventimiglia blocked the dopamine-responding neurons, the flies continued to fight. This work suggests that the dopamine neurons are signaling something akin to what humans experience as fear, and shutting the neurons down prevents the flies from getting the message to avoid potentially dangerous situations.
“I don’t know whether we should be calling this behavior ‘fear,’” says Asahina cautiously, “but there are clear behavioral changes after this social-defeat experience, and maybe what’s happening in the fly brain has some similarity to what people call a fear response.”

Assistant Professor Sung Han, who holds the Pioneer Fund Developmental Chair, is an expert on the fear response. Using mouse models, he studies small chemicals known as neuropeptides and how they influence the brain’s built-in alarm system. This system first recognizes environmental threats detected by the senses, and then issues commands to change physiology, metabolism, behaviors, and emotions to avoid these threats.
“For example, if you smell smoke, then see flames and feel heat, you understand that there’s a fire and you need to get away,” Han says.
Earlier this year, Han, postdoctoral researcher Sukjae Joshua Kang, and graduate student Shijia Liu uncovered a molecular pathway that distills such threatening smells, sights, and sensations into a single warning. The team discovered that a molecule called CGRP enables neurons in two separate areas of the brain (the thalamus and brainstem) to bundle threatening sensory cues into a unified signal, tag it as negative, and convey it to the amygdala—which translates the threat signal into the feeling of fear.
“But, just as with house or car security technology, the biological alarm system can malfunction and generate false alarms,” Han says.
“But, just as with house or car security technology, the biological alarm system can malfunction and generate false alarms.”
– Sung Han

This hypersensitivity to otherwise normal sensory stimuli is linked to PTSD, panic disorders, anxiety disorder, chronic pain disorder, schizophrenia, and autism spectrum disorders.
The same brain regions that express CGRP in mice also do so in humans. Han’s team is now eager to study how the molecule might be involved in hypersensitivity disorders, and whether a drug that blocks the molecule could be helpful in relieving symptoms.
Hypersensitivity disorders often involve our sense of touch—people with autism may not like certain textures, while people with neuropathic pain may find light touch painful. Touch sense is a focus for Associate Professor Eiman Azim, who aims to piece together the neural underpinnings of movement, especially skilled motions like reaching, grasping, and manipulating objects.
Recently, Azim, who holds the William Scandling Developmental Chair, and graduate student Jacqueline Mosko became interested in how our internal states—including emotions—might influence touch sensitivity.
“For example, fear can be associated with greater sensitivity to touch, which can be beneficial, in the short term, in scenarios where one needs to be exquisitely aware of details about the environment,” Azim says.
This change in touch sensitivity, however, can be problematic when it becomes chronic. Preliminary work suggests that the stress of prolonged social isolation, in addition to affecting our mental health, can lead to aberrant sensitivity to touch.
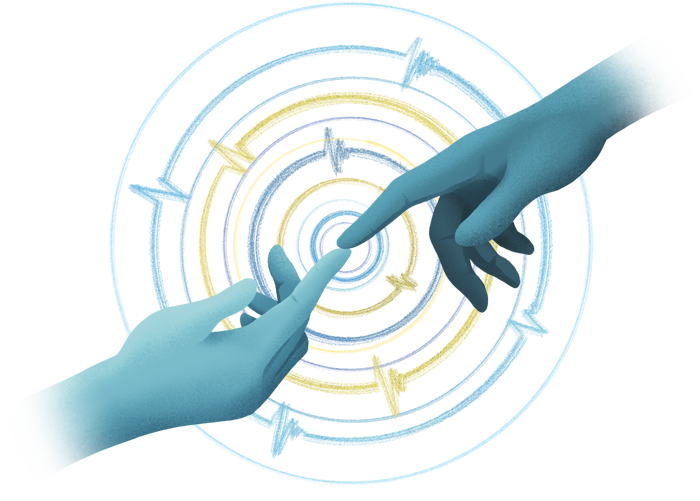
“So now we want to understand what is actually happening in the brain to affect touch sensitivity following social isolation.”
– Eiman Azim
The team’s preliminary experiments in mice suggest that regulation of touch processing in an evolutionarily ancient structure in the brainstem, the cuneate nucleus, might be key.
Azim and staff researcher James Conner recently published a study about the cuneate nucleus showing that this structure—previously thought to be only a relay station for sensory information coming from the body—actually helps filter the information before routing it to other brain areas. This filtering is important for preventing distracting sensations from interfering with relevant ones. (When your fingers are typing an email, for example, you don’t need to be aware of your sleeve brushing your arm.)
Azim, Mosko, and Conner wonder if this filtering is disrupted by the stress of social isolation, leading to hypersensitivity to touch. In this scenario, typically innocuous sensations, like the feel of clothes touching skin, could become distracting or unpleasant and could even evoke abnormal behaviors such as avoidance of touch. The team is currently conducting experiments to test their hypothesis and exploring any implications their work might have for remedies.
Social isolation in and of itself is not necessarily a bad thing, Salk Professor and President Rusty Gage points out.
“Short-term isolation that’s by choice can be refreshing—a meditation retreat, for example, or a backpacking trip,” he says. “The problem is when isolation becomes chronic and isn’t by choice that the brain can begin rewiring itself in harmful ways.”
Gage, a neuroscientist, is perhaps best known for discovering that the brain continues to make new neurons in adulthood, a process called neurogenesis.
“New neurons help with familiarity—knowing if you’ve met someone before—and familiarity is very helpful in social situations,” Gage says.
But, he says, chronic stress decreases the brain’s ability to generate neurons, especially in a region of the brain known as the hippocampus, which recognizes and makes distinctions between events, emotions, objects, and people.
“Physical exercise is therapeutic for depression, for anxiety, for traumatic brain injury. It reduces the risk of dementia.”
– Rusty Gage
Fortunately, there is a way to promote neurogenesis to improve health and well-being, and perhaps even help alleviate health issues caused by social isolation and chronic stress.
“One of the best things people can do for neurological health is exercise, because it gets blood flowing,” says Gage, who holds the Vi and John Adler Chair for Research on Age-Related Neurodegenerative Disease. “Physical exercise is therapeutic for depression, for anxiety, for traumatic brain injury. It reduces the risk of dementia.”
This also applies for people with autism, who have a decreased capacity for social interaction, which Gage says is itself isolating. “Some of the best behavioral therapy for autism is finding the right vehicle of communication with the autistic individual so that they can do things that help them get authentically reinforcing rewards. What does a person with autism find enjoyable? A lot of times it’s repetitive movements. There are repetitive computer activities they can do that are actively stimulating. And then if you can build physical exercise into an enriched environment, that’s even better.”
Gage says the pandemic has made society acutely aware of the negative consequences of social isolation on mental health, and of the importance of research into the molecular mechanisms that result from it.
“At Salk, the important part is getting people from different perspectives coming together and discussing the issue. Coming up with animal models, coming up with mathematical models, ways in which we can generate a hypothesis about what’s happening and test it—in a way that’s translatable to humans—so we know how to best modify our environments or reset ourselves to build resilience and cope better in our ever-changing world.”
[ssba]
Support a legacy where cures begin.
Featured Stories
 Human Connection – How social interaction and isolation influence our physical and mental healthSalk neuroscientists study how our brains allow us to experience, interpret, and interact with the world around us. Many of these researchers are now studying not only the brain alone, but the brain in a social context.
Human Connection – How social interaction and isolation influence our physical and mental healthSalk neuroscientists study how our brains allow us to experience, interpret, and interact with the world around us. Many of these researchers are now studying not only the brain alone, but the brain in a social context. Gerald Joyce—An organizing forceJoyce was appointed Salk’s senior vice president and chief science officer in early 2022. Inside Salk sat down with him to learn about his research on the evolution of RNA, as well as the evolution of his career, science, and Salk.
Gerald Joyce—An organizing forceJoyce was appointed Salk’s senior vice president and chief science officer in early 2022. Inside Salk sat down with him to learn about his research on the evolution of RNA, as well as the evolution of his career, science, and Salk.
 Salk scientists lead $126 million effort to map the aging human brainThe largest grant in Institute history has established the new Center for Multiomic Human Brain Cell Atlas to detail the many individual cells that make up the human brain—their molecular features, where they are found, and how they change with age.
Salk scientists lead $126 million effort to map the aging human brainThe largest grant in Institute history has established the new Center for Multiomic Human Brain Cell Atlas to detail the many individual cells that make up the human brain—their molecular features, where they are found, and how they change with age. A life in service to science and others: Walter Eckhart exemplified generosity and kindnessEckhart, professor emeritus and director of the Salk Institute’s National Cancer Institute-designated Cancer Center and head of the Molecular and Cell Biology Laboratory for more than 30 years, died on June 21, 2022, at his home in La Jolla, California, at the age of 84.
A life in service to science and others: Walter Eckhart exemplified generosity and kindnessEckhart, professor emeritus and director of the Salk Institute’s National Cancer Institute-designated Cancer Center and head of the Molecular and Cell Biology Laboratory for more than 30 years, died on June 21, 2022, at his home in La Jolla, California, at the age of 84.  Georg Heinrich “Heini” Thyssen-Bornemisza—Salk Institute mourns loss of influential former Board memberThyssen’s leadership and generosity helped accelerate scientific efforts at the Institute over the years, always with a focus on allowing Salk scientists to continue their pursuit of high-risk, high-impact research. He died on September 30, 2022.
Georg Heinrich “Heini” Thyssen-Bornemisza—Salk Institute mourns loss of influential former Board memberThyssen’s leadership and generosity helped accelerate scientific efforts at the Institute over the years, always with a focus on allowing Salk scientists to continue their pursuit of high-risk, high-impact research. He died on September 30, 2022. Mallory Zaslav—Valuing the differences in backgrounds and experiencesAs vice president of Diversity, Equity & Inclusion, Zaslav’s forward thinking and advocacy have given shape to a range of impact-driven programming and outreach, furthering Salk’s mission of bettering humanity by pushing the boundaries of innovation and discovery.
Mallory Zaslav—Valuing the differences in backgrounds and experiencesAs vice president of Diversity, Equity & Inclusion, Zaslav’s forward thinking and advocacy have given shape to a range of impact-driven programming and outreach, furthering Salk’s mission of bettering humanity by pushing the boundaries of innovation and discovery. Katia Troha—Discovering diets that boost survival during infectionGrowing up in Peru, Troha’s love of science was fueled by documentaries on groundbreaking studies like the first cloned mammal, Dolly the sheep. She is now a postdoctoral researcher in the Salk lab of Professor Janelle Ayres.
Katia Troha—Discovering diets that boost survival during infectionGrowing up in Peru, Troha’s love of science was fueled by documentaries on groundbreaking studies like the first cloned mammal, Dolly the sheep. She is now a postdoctoral researcher in the Salk lab of Professor Janelle Ayres. Heithoff-Brody High School Summer Scholars program paves the way for future scientistsFor more than 30 years, Salk’s Heithoff-Brody High School Summer Scholars program has provided hands-on laboratory experiences for local high school students interested in exploring careers in science, technology, engineering, and math.
Heithoff-Brody High School Summer Scholars program paves the way for future scientistsFor more than 30 years, Salk’s Heithoff-Brody High School Summer Scholars program has provided hands-on laboratory experiences for local high school students interested in exploring careers in science, technology, engineering, and math.





















































